Efficacy and immunogenicity of rKVAC85B in a BCG prime-boost regimen against H37Rv and HN878 Mycobacterium tuberculosis strains
- PMID: 40367100
- PMCID: PMC12077692
- DOI: 10.1371/journal.pone.0322147
Efficacy and immunogenicity of rKVAC85B in a BCG prime-boost regimen against H37Rv and HN878 Mycobacterium tuberculosis strains
Abstract
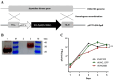
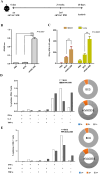
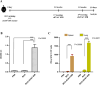
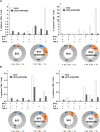
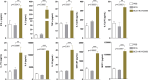
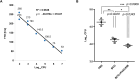
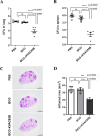
Mycobacterium tuberculosis infection accounted for 1.3 million deaths worldwide in 2022. Bacillus Calmette-Guérin (BCG) is the only licensed vaccine against tuberculosis (TB); however, it has limited protective efficacy in adults. In this study, we constructed a recombinant vaccinia virus expressing Ag85B from M. tuberculosis using a novel attenuated vaccinia virus (KVAC103). We then analyzed the immunogenicity of prime-boost inoculation strategies using recombinant KVAC103 expressing Ag85B (rKVAC85B) compared to BCG. In both rKVAC85B prime-boost and BCG prime-rKVAC85B boost inoculation regimens, rKVAC85B induced the generation of specific immunoglobulin G (IgG) and secretion of interferon-γ by immune cells. In vitro analysis of Mycobacterium growth inhibition revealed a comparable immune-mediated pattern of outcomes. Furthermore, bacterial loads in the lungs were significantly lower in mice inoculated with the BCG prime-rKVAC85B boost than in the BCG-only group following a rechallenge infection with both H37Rv and HN878 strains of M. tuberculosis. These findings collectively suggest that KVAC103, incorporated into a viral vector, is a promising candidate for the development of a novel TB vaccine platform that is effective against multiple M. tuberculosis strains, including H37Rv and HN878, and that rKVAC85B effectively stimulates immune responses against M. tuberculosis infection.
Copyright: © 2025 Shin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Global tuberculosis report 2023. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2023.
-
- Talbot EA, Silva SFM, Frothingham R. Disseminated Bacille Calmette-Guérin Disease After Vaccination: Case Report and Review. Clinical Infectious Diseases. 1997;24:1139–46. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

