The role of dopaminergic medication and specific pathway alterations in idiopathic and PRKN/PINK1-mediated Parkinson's disease
- PMID: 40367158
- PMCID: PMC12077494
- DOI: 10.1126/sciadv.adp7063
The role of dopaminergic medication and specific pathway alterations in idiopathic and PRKN/PINK1-mediated Parkinson's disease
Abstract
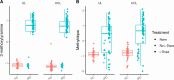
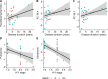
Parkinson's disease (PD) is the second most common neurodegenerative disease, with a rapidly increasing prevalence worldwide. Biomarkers monitoring state and progression are urgently needed, and metabolomics from easily accessible biofluids holds the potential to elucidate pathophysiological underpinnings in PD. Several studies suggested metabolomic differences between patients and controls, but findings are controversial, and independent replication is scarce. We thus applied state-of-the-art, large-scale metabolomics in patients with idiopathic and monogenic PD and controls from two independent samples, analyzed by a strict meta-analysis approach. Thereby, we (i) debunked that l-Dopa medication and not disease status causes the most substantial metabolomic differences and (ii) identified polyamine metabolism alterations, partly, but not entirely associated with l-Dopa treatment. Furthermore, we found explorative but robust evidence for alterations in endocannabinoid metabolites; detected lipid metabolism alterations, highlighting potential crosslinks with alpha-synuclein pathology; and provided evidence for a metabolomic signature for the role of oxidative damage in patients with PRKN- and PINK1-linked PD.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

