Development of potent humanized TNFα inhibitory nanobodies for therapeutic applications in TNFα-mediated diseases
- PMID: 40367237
- PMCID: PMC12080732
- DOI: 10.1080/19420862.2025.2498164
Development of potent humanized TNFα inhibitory nanobodies for therapeutic applications in TNFα-mediated diseases
Abstract
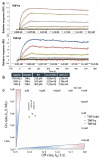
Tumor necrosis factor-alpha (TNFα) is a key pro-inflammatory cytokine implicated in the pathogenesis of numerous inflammatory and autoimmune diseases, including rheumatoid arthritis, inflammatory bowel disease, and neurodegenerative disorders such as Alzheimer's disease. Effective inhibition of TNFα is essential for mitigating disease progression and improving patient outcomes. In this study, we present the development and comprehensive characterization of potent humanized TNFα inhibitory nanobodies (TNFI-Nbs) derived from camelid single-domain antibodies. In silico analysis of the original camelid nanobodies revealed low immunogenicity, which was further reduced through machine learning-guided humanization and developability optimization. The two humanized TNFI-Nb variants we developed demonstrated high anti-TNFα activity, achieving IC₅₀ values in the picomolar range. Binding assays confirmed their high affinity for TNFα, underscoring robust neutralization capabilities. These TNFI-Nbs present valid alternatives to conventional monoclonal antibodies currently used in human therapy, offering potential advantages in potency, specificity, and reduced immunogenicity. Our findings establish a solid foundation for further preclinical development and clinical translation of TNFα-targeted nanobody therapies in TNFα-mediated diseases.
Keywords: Alzheimer disease; TNFα; TNFα inhibition; nanobody; nanobody humanization; single-domain antibody.
Conflict of interest statement
Dr. Luciano D’Adamio is a Professor at Rutgers University and also the founder of NanoNewron, a biotechnology company focused on developing innovative therapeutics for central nervous system diseases. The contribution to this study of Pietro Sormanni, Aubin Ramon and Matthew Greenig was conducted in a consultancy capacity and was remunerated by NanoNewron LLC.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
