Modeling protective meningococcal antibody responses and factors influencing antibody persistence following vaccination with MenAfriVac using machine learning
- PMID: 40367245
- PMCID: PMC12077764
- DOI: 10.1371/journal.pone.0323384
Modeling protective meningococcal antibody responses and factors influencing antibody persistence following vaccination with MenAfriVac using machine learning
Abstract
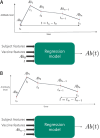
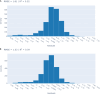
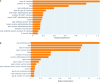
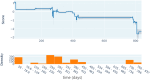
Meningococcal meningitis poses a significant public health burden in the meningitis belt region of sub-Saharan Africa. The introduction of the meningococcal PsA-TT vaccine (MenAfriVac®) has successfully eliminated Neisseria meningitidis serogroup A (NmA) cases in the region. However, the duration of post-vaccination immunity and the need for booster doses remain uncertain. To address this knowledge gap, we developed computational models using machine learning techniques to improve the effectiveness of modeling in guiding vaccination strategies for the African meningitis belt. Using serologic data from previous clinical trials of PsA-TT, we proposed a short-term and a long-term model that integrated demographic and medical variables (such as age, height and weight) with previous antibody titer levels and vaccination information to predict NmA antibody titer levels following vaccination. In the short-term model, we found moderately high performance (R-squared = 0.59) for out-of-training-data subjects and even better performance (R squared = 0.83) in the long-term evaluation. Our models estimated the half-life of the vaccine to be 13.9 years for the study population overall, similar to previously reported estimates. Machine learning techniques offer several advantages over previous approaches, as they do not require multiple readings from the same subject, can be rigorously validated using a subset of subject data not used for training. The proposed approach also facilitates the interpretation of the relationship between input variables and antibody levels at a population level. By incorporating subject-specific demographic and medical variables, our models could potentially be used to tailor vaccination schedules to at-risk populations.
Copyright: © 2025 Nasir et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

