Drop Friction and Failure on Superhydrophobic and Slippery Surfaces
- PMID: 40367354
- PMCID: PMC12120990
- DOI: 10.1021/acsnano.5c01142
Drop Friction and Failure on Superhydrophobic and Slippery Surfaces
Abstract
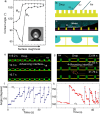
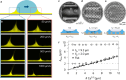
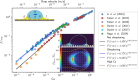
The mobility of drops on a surface influences how much water and energy is required to clean the surface. By controlling drop mobility, it is possible to promote or reduce fogging, icing, and fouling. Superhydrophobic and slippery liquid-infused surfaces both display high drop mobility despite being 'lubricated' by fluids having very different viscosities. Superhydrophobic surfaces rely on micro- and/or nanoscale textures to trap air pockets beneath drops, minimizing solid-liquid contact. In contrast, on liquid-infused surfaces, these solid textures are filled with an immiscible liquid lubricant. Over the past few years, innovations in experimental and computational methods have provided detailed new insights into the static and dynamic wetting properties of drops on these surfaces. In this review, we describe the criteria needed to obtain stable wetting states with low drop friction and high mobility on both surfaces, and discuss the mechanisms that have been proposed to explain the origins of friction on each surface. Drops can collapse from the low-friction Cassie state to the high-friction Wenzel state on both surfaces, but the transition follows different pathways: on liquid-infused surfaces, the wetting ridge near the drop edge plays a central role in triggering collapse, a phenomenon not observed on superhydrophobic surfaces. This review emphasizes that a liquid-infused surface cannot be simply viewed as a superhydrophobic surface with the air pockets replaced by lubricant. The wetting ridge surrounding drops on liquid-infused surfaces significantly alters most of the drop's properties, including macroscopic shape, friction mechanisms, and the mechanism of collapse to a Wenzel state.
Keywords: adhesion; capillarity; drops; friction; interfacial phenomena; lubrication; roughness; surface cleaning; wetting.
Figures









References
-
- de Gennes, P.-G. ; Brochard-Wyart, F. ; Quéré, D. . Capillarity and Wetting Phenomena, 1st ed.; Springer: New York, NY, 2004. 10.1007/978-0-387-21656-0. - DOI
-
- Bormashenko, E. Yu. Wetting of Real Surfaces; De Gruyter: Berlin, Boston, 2018. 10.1515/9783110583144. - DOI
-
- Butt, H.-J. ; Kappl, M. . Surface and Interfacial Forces, 2nd ed.; Wiley-VCH, 2018.
-
- Wagner T., Neinhuis C., Barthlott W.. Wettability and Contaminability of Insect Wings as a Function of Their Surface Sculptures. Acta Zool. 1996;77:213–225. doi: 10.1111/j.1463-6395.1996.tb01265.x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

