Pain in fibrous dysplasia: identifying nociceptive mechanisms in a preclinical model
- PMID: 40367355
- PMCID: PMC12188752
- DOI: 10.1093/jbmr/zjaf039
Pain in fibrous dysplasia: identifying nociceptive mechanisms in a preclinical model
Abstract
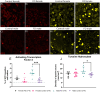
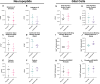
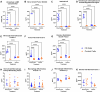
Pain is a common symptom of fibrous dysplasia (FD), a rare mosaic disorder characterized by fibro-osseous lesions in the bone. Despite the prevalence of pain in FD patients, there is little knowledge about the nociceptive mechanisms and few efficacious treatments. As such, understanding FD pain is essential for patient care. The overall aim of this study was to identify nocifensive behaviors and potential underlying mechanisms in a transgenic mouse model of FD, previously shown to display high face and translational validity. Significant nocifensive behaviors were observed in FD mice (male and female), compared to control mice in the burrowing, grid hanging, home cage activity, and wheel running assays. These changes corresponded to lesion development, as visualized by X-ray imaging. Behavioral deficits improved when analgesics were administered, indicating a nociceptive origin. Tibias and femurs from FD mice demonstrated characteristic FD lesions and the presence of mono- and multi-nucleated CD68+ cells, calcitonin gene-related peptide sensory nerve fibers, and vascularization. Lumbar dorsal root ganglia from male FD mice displayed increased staining for activating transcription factor-3 and tyrosine hydroxylase neurons. No difference was observed in the spinal cords between the FD and control groups for glial cell presence and neuropeptide expression. Bone marrow stromal cells were obtained from FD and control mice and cultured in vitro. FD cells developed an increased concentration of inflammatory cytokines (IL-6, tumor necrosis factor-alpha), chemokines (monocyte chemoattractant protein, keratinocyte chemoattractant/human growth-regulated oncogene), and nerve growth factor as compared to controls. Taken together, this study demonstrated for the first time that nociceptive mechanisms such as axonal growth in FD lesions, nerve injury, and inflammation may contribute to FD pain, and it provides a foundation for conducting further studies of pain- and disease-modifying therapeutics for FD patients.
Keywords: bone pain; fibrous dysplasia; in vivo; mechanism; pain.
Plain language summary
Fibrous dysplasia (FD) is a rare and often painful bone disease. Although pain is a key concern for the patients, the treatment is often inadequate, due in part to the lack of knowledge of the underlying mechanisms. Using a mouse model of FD, we found that behavioral measures such as burrowing, grid hanging, cage activity, and wheel running decreased when FD developed. Furthermore, burrowing and grid hanging improved when analgesics were administered, which suggests that reduced behavior is related to pain. By assessing the bones, we discovered alterations of the FD microenvironment, the presence of nerve fibers (including sensory axons) in the FD lesions, and evidence of nerve damage in the peripheral nervous system. Cells taken from the mouse bones were shown to secrete pro-inflammatory proteins and nerve growth factors from FD cells. This is the first study to characterize FD pain mechanisms. Taken together, the results suggest several key mechanisms underlying FD pain and it may lead to a more targeted and effective pain treatment for FD patients.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
None declared.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

