Polystyrene Microplastics-Induced Thyroid Dysfunction in Mice: A Study of Gene Expression, Oxidative Stress, and Histopathological Changes
- PMID: 40367361
- PMCID: PMC12077756
- DOI: 10.1002/vms3.70393
Polystyrene Microplastics-Induced Thyroid Dysfunction in Mice: A Study of Gene Expression, Oxidative Stress, and Histopathological Changes
Abstract
Background: Polystyrene microplastics (PS-MPs) are pervasive pollutants impacting animals across ecosystems, including livestock and wildlife, through contaminated food, water, and air. MPs may disrupt endocrine function, particularly affecting the thyroid gland, which is essential for metabolism and development.
Objectives: This study investigates the effects of PS-MPs on thyroid function in mice, offering insights relevant to veterinary care by examining changes in gene expression and biochemical markers.
Methods: PS-MPs of 5 µm diameter were prepared in distilled water after probe sonication. Sixty male Swiss albino mice were divided into three groups: a control group and two treatment groups receiving 0.1 mg and 0.2 mg PS-MPs via oral gavage for 28 days. Mice were anesthetised, and thyroid tissues were collected for histopathological, biochemical, and gene expression analyses. Biochemical tests included catalase, superoxide dismutase, reactive oxygen species, and hormone levels. Histopathology and gene expression (TSHR and TPO) of thyroid-related genes were examined to assess PS-MPs induced effects.
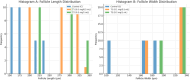
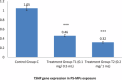
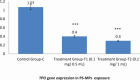
Results: Exposure to PS-MPs in mice led to significant increases in calcium, thyroxin, free T3, free T4, ALP, AST, ALT, and amylase levels, alongside elevated oxidative stress markers. Conversely, the levels of TSH, calcitonin, magnesium and phosphate decreased. Histopathological analysis showed abnormal thyroid follicle development, decrease parafollicular cells, with colloid loss, haemorrhage, and necrosis. Gene expression analysis revealed a marked reduction in TSHR and TPO levels in PS-MPs treated groups, indicating thyroid dysfunction. These findings highlight the profound impact of PS-MPs on thyroid gland function in mice.
Conclusion: These findings underscore the potential risks that PS-MPs pose to thyroid health, with potential consequences for other veterinary species. As environmental contamination rises, veterinarians may encounter more endocrine disorders linked to PS-MPs, emphasising the need for further research and preventive measures.
Keywords: adverse effects; mice; microplastics; thyroid function.
© 2025 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Al‐Thawadi, S. 2020. “Microplastics and Nanoplastics in Aquatic Environments: Challenges and Threats to Aquatic Organisms.” Arab Journal of Science and Engineering 45: 4419–4440. 10.1007/s13369-020-04402-z. - DOI
-
- Amereh, F. , Eslami A., Fazelipour S., Rafiee M., Zibaiid M. I., and Babaei M.. 2019. “Thyroid Endocrine Status and Biochemical Stress Responses in Adult Male Wistar Rats Chronically Exposed to Pristine Polystyrene Nanoplastics.” Toxicology Research 8, no. 6: 931–942. 10.1039/c9tx00147f. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

