Second-Line Antiretroviral Therapy for Children Living with HIV in Africa
- PMID: 40367375
- PMCID: PMC7617794
- DOI: 10.1056/NEJMoa2404597
Second-Line Antiretroviral Therapy for Children Living with HIV in Africa
Abstract
Background: Children living with human immunodeficiency virus (HIV) have limited options for second-line antiretroviral therapy (ART).
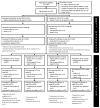
Methods: In this open-label trial with a 2-by-4 factorial design, we randomly assigned children with HIV who had first-line treatment failure to receive second-line therapy with tenofovir alafenamide fumarate (TAF)-emtricitabine or standard care (abacavir or zidovudine, plus lamivudine) as the backbone and dolutegravir or ritonavir-boosted darunavir, atazanavir, or lopinavir as the anchor drug. The primary outcome was a viral load of less than 400 copies per milliliter at 96 weeks. We hypothesized that TAF-emtricitabine would be noninferior to standard care, that dolutegravir and ritonavir-boosted darunavir would each be superior to ritonavir-boosted lopinavir and atazanavir analyzed in combination, and that ritonavir-boosted atazanavir would be noninferior to ritonavir-boosted lopinavir. Safety was also assessed.
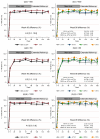
Results: A total of 919 children underwent randomization; 458 were assigned to receive TAF-emtricitabine, and 461 to receive standard care. Assigned anchor drugs were dolutegravir (229 participants), ritonavir-boosted darunavir (232), ritonavir-boosted atazanavir (231), and ritonavir-boosted lopinavir (227). The median age of participants was 10 years, and 497 (54.1%) were male. The median viral load at baseline was 17,573 copies per milliliter. At week 96, TAF-emtricitabine was superior to standard care: the adjusted difference in the percentage of participants with a viral load of less than 400 copies per milliliter was 6.3 percentage points (95% confidence interval [CI], 2.0 to 10.6; P = 0.004). Dolutegravir was superior to ritonavir-boosted lopinavir and atazanavir analyzed in combination (adjusted difference, 9.7 percentage points; 95% CI, 4.8 to 14.5; P<0.001), but ritonavir-boosted darunavir was not (adjusted difference, 5.6 percentage points; 95% CI, 0.3 to 11.0; P = 0.04 [prespecified threshold, P = 0.03]). Ritonavir-boosted atazanavir was noninferior to ritonavir-boosted lopinavir. One child died, and 29 (3.2%) had serious adverse events, with no significant between-group differences.
Conclusions: Second-line ART regimens including TAF-emtricitabine and dolutegravir were effective for children, with no evidence of safety concerns. Ritonavir-boosted darunavir was also effective. (Funded by the European and Developing Countries Clinical Trials Partnership and others; CHAPAS-4 ISRCTN Registry number, ISRCTN22964075.).
Copyright © 2025 Massachusetts Medical Society.
Figures

References
-
- Njom-Nlend A-E, Efouba N, Brunelle Sandie A, Fokam J. Determinants of switch to paediatric second-line antiretroviral therapy after first-line failure in Cameroon. Tropical Medicine & International Health. 2021;26:927–35. - PubMed
-
- The Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration. Incidence of switching to second-line antiretroviral therapy and associated factors in children with HIV: an international cohort collaboration. Lancet HIV. 2019;6(6):e105–e115. doi: 10.1016/S2352-3018(18)30319-9. - DOI - PMC - PubMed
-
- Mulenga L, Fwoloshi S, Mweemba A, et al. Dolutegravir with recycled NRTIs is noninferior to PI-based ART: VISEND trial; CROI; 12–16 February; 2022. Oral abstract ( https://www.croiconference.org/abstract/dolutegravir-with-recycled-nrtis...)
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- TRIA2015-1078/European and Developing Countries Clinical Trials Partnership
- CO-EU-311-4461/Gilead Sciences
- RIA2019PD-2882/European and Developing Countries Clinical Trials Partnership
- RIA2019PD-2882/EDCTP_/European & Developing Countries Clinical Trials Partnership (EDCTP)/Netherlands
- TRIA2015-1078/EDCTP_/European & Developing Countries Clinical Trials Partnership (EDCTP)/Netherlands
LinkOut - more resources
Full Text Sources
Medical
