Profiling human iPSC-derived sensory neurons for analgesic drug screening using a multi-electrode array
- PMID: 40367946
- PMCID: PMC12146644
- DOI: 10.1016/j.crmeth.2025.101051
Profiling human iPSC-derived sensory neurons for analgesic drug screening using a multi-electrode array
Abstract
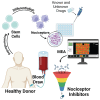
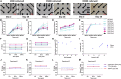
Chronic pain is a global health issue, yet effective treatments remain limited due to poor preclinical-to-human translation. To address this, we developed a high-content screening (HCS) platform using hiPSC-derived nociceptors to identify analgesics targeting the peripheral nervous system. These cells, cultured on multi-well microelectrode arrays, achieved nearly 100% active electrodes by week 2, maintaining stable activity for at least 2 weeks. After 28 days, we assessed drug effects on neuronal activity, achieving strong assay performance (robust Z' > 0.5). Pharmacological tests confirmed responses to key analgesic targets, including ion channels (Nav, Cav, Kv, and TRPV1), neurotransmitter receptors (AMPAR and GABA-R), and kinase inhibitors (tyrosine and JAK1/2). Transcriptomic analysis validated target expression, though levels differed from primary human DRG cells. The platform was used to screen over 700 natural compounds, demonstrating its potential for analgesic discovery. This HCS platform facilitates the rapid discovery of uncharacterized analgesics, reducing preclinical-to-human translation failure.
Keywords: CP: Stem cell; DRG; analgesic discovery; chronic pain; hiPSC; high-content screening; human induced pluripotent stem cell; nociceptor.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.W. and V.T. are shareholders and employees of Anatomic Incorporated. The hiPSC nociceptors “RealDRG” and associated reagents were provided by Anatomic for the project through co-investigator status on R61/R33 awards.
Figures





Update of
-
Profiling Human iPSC-Derived Sensory Neurons for Analgesic Drug Screening Using a Multi-Electrode Array.bioRxiv [Preprint]. 2024 Nov 18:2024.11.18.623405. doi: 10.1101/2024.11.18.623405. bioRxiv. 2024. Update in: Cell Rep Methods. 2025 May 19;5(5):101051. doi: 10.1016/j.crmeth.2025.101051. PMID: 39605708 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

