Selection of patient-reported outcome measures in pulmonary arterial hypertension clinical trials: a systematic review, meta-analysis and health-related quality of life framework
- PMID: 40368429
- PMCID: PMC12076161
- DOI: 10.1183/16000617.0006-2025
Selection of patient-reported outcome measures in pulmonary arterial hypertension clinical trials: a systematic review, meta-analysis and health-related quality of life framework
Abstract
Introduction: Health-related quality of life (HRQoL) in pulmonary arterial hypertension (PAH) is valued as an outcome measure by patients, clinicians and regulators. The selection of patient-reported outcome measures (PROMs) for measurement of HRQoL in PAH clinical trials lacks systematic evaluation of their suitability, accuracy and reliability.
Methods: We report a systematic review (PROSPERO ID: CRD42024484021) following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of PROMs selected in PAH clinical trials. PROM measurement properties were then evaluated according to the 10-step COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist and graded by recommendation for use. Finally, HRQoL was modelled into a conceptual framework using patient interviews and surveys.
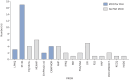
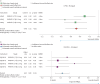
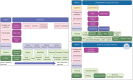
Results: Screening of 896 records identified 90 randomised controlled trials. 43 trials selected PROMs, of which 20 were sufficiently validated to detect meaningful change. Of these, eight trials were adequately powered, using either EuroQol-five dimensions-five levels (EQ-5D-5L), Short-Form-36 (SF-36) or the Living with Pulmonary Hypertension Questionnaire (LPHQ). The COSMIN evaluation recommended EmPHasis-10 and the LPHQ for use (grade A); whereas, SF-36 and EQ-5D-5L require further study (grade B). A conceptual framework of HRQoL was developed from literature comprising 8045 patients. This framework can be used to visualise the different HRQoL concepts measured by different PROMs.
Conclusion: To improve patient-centred research, greater consistency in PROM selection is required. Three of 90 randomised controlled trials have selected COSMIN-recommended PROMs. Whilst the PROMs evaluated require development across the 10 areas of psychometric property measurement, EmPHasis-10 and the LPHQ can be recommended for use. The ratified conceptual framework can further support PROM selection by identifying the HRQoL concepts they are likely to capture.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: D.G. Kiely and I. Armstrong were involved in the derivation of EmPHasis-10 but remained independent in the risk of bias analysis for the COSMIN review. J. Carlton is a co-investigator for the UK EQ-5D-5L study team. T. Peasgood is a member of EuroQol and involved in research development of the EuroQol Health and Wellbeing instrument. The other authors are not affiliated with PROMs evaluated in this review. The authors (A.M.K. Rothman/F. Varian/R. Burney/C. Pearson/Z.M. Goh/J. Newman) performing the data collection, selection and evaluation of review articles have no conflicts of interest. A.A.R. Thompson reports research funding from Heart Research UK, Janssen-Cilang Ltd, British Heart Foundation, and honoraria from Janssen-Cilad Ltd for lectures and education. A.M.K. Rothman reports research funding from Wellcome Trust Clinical Research Career Development Fellowship (206632/Z/17/Z), Medical Research Council (UK) Experimental Medicine Award (MR/W026279/1) and NIHR Biomedical Research Centre Sheffield; contributions in kind from Medtronic Inc., Abbott Laboratories, Endotronix Inc., Novartis, Janssen and Merk; and research support and consulting from NXT Biomedical, Endotronix Inc., SoniVie, Neptune and Gradient. D.G. Kiely has received personal funding from the NIHR Biomedical Research Centre Sheffield, research funding from Ferrer, GSK and Janssen, and consulting and educational funding from Acceleron, Altivant, Ferrer, Gossamer, Janssen, MSD and United Therapeutics. F. Varian has received educational funding from Janssen and is a Medical Research Council (UK) clinical fellow. J. Newman reports research funding from British Heart Foundation, and education and travel funding from Aparito Ltd and United Therapeutics. M. Toshner reports research funding from NIHR Biomedical Research Centre Cambridge and NIHR HTA; and personal support from GSK and Jansen. R. Condliffe has received honoraria for speakers’ fees and conference travel from Janssen. The remaining authors have no conflicts to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
