Interleukin-1 receptor antagonist polymorphisms in women receiving epidural analgesia who develop maternal intrapartum fever: a prospective, multicentre Mendelian randomised study
- PMID: 40368685
- PMCID: PMC12226748
- DOI: 10.1016/j.bja.2025.03.037
Interleukin-1 receptor antagonist polymorphisms in women receiving epidural analgesia who develop maternal intrapartum fever: a prospective, multicentre Mendelian randomised study
Abstract
Background: Genetically predicted higher levels of the anti-inflammatory cytokine interleukin-1 receptor antagonist (IL1-Ra) might reduce the risk of developing epidural-related maternal fever, a phenomenon that occurs exclusively in women having epidural analgesia in labour. We hypothesised that in women having epidural analgesia, the absence of specific alleles that lower circulating levels of IL1-Ra would be associated with the development of epidural-related maternal fever, administration of intrapartum antibiotics, or both.
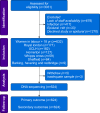
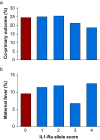
Methods: We prospectively enrolled women ≥18 yr of age receiving epidural analgesia during labour, excluding those with pre-existing fever, antibiotic therapy, or immunodeficiency. Allele scores were constructed from genotyping the C-allele frequency at variants rs6743376 and rs1542176; more copies of each allele independently raise IL-1Ra. The composite primary outcome was maternal intrapartum fever (>38°C) or administration of intrapartum antibiotics after epidural placement. The exposure of interest was the IL1-Ra allele score, comparing 0 (lowest genetically predicted IL-1Ra levels) with ≥1 allele scores. Maternal fever and antibiotic administration were compared in women with 0 or ≥1 allele scores.
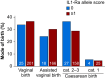
Results: Of 624 women genotyped, 155 (24.8%) developed maternal fever or received antibiotics. Fever or antibiotic administration occurred in 19/74 (25.7%) labouring women with an IL-1Ra allele score of 0, compared with 136/550 (24.7%) women with IL-1Ra allele scores ≥1 (odds ratio 1.05, 95% confidence interval 0.60-1.83; P=0.89).
Conclusions: In women who receive epidural analgesia during labour, genetically predicted (higher) interleukin-1 receptor antagonist levels do not alter the incidence of maternal intrapartum fever or use of intrapartum antibiotics.
Clinical trial registration: ISRCTN99641204.
Keywords: Mendelian randomisation; epidural; fever; genetics; inflammation; interleukin-1 receptor antagonist; intrapartum.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interest TEFA and GLA are editorial board members of the British Journal of Anaesthesia.
Figures

References
-
- NHS Maternity Statistics, England, 2023–24. NHS England Digital. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/nhs... (accessed December 24, 2024)
-
- Ayres-de-Campos D., Spong C.Y., Chandraharan E., Panel F.I.F.M.E.C. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynaecol Obstet. 2015;131:13–24. - PubMed
-
- Patel S., Ciechanowicz S., Blumenfeld Y.J., Sultan P. Epidural-related maternal fever: incidence, pathophysiology, outcomes, and management. Am J Obstet Gynecol. 2023;228:S1283–S1304 e1. - PubMed
-
- Sultan P., David A.L., Fernando R., Ackland G.L. Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg. 2016;122:1546–1553. - PubMed
-
- Del Arroyo A.G., Sanchez J., Patel S., et al. Role of leucocyte caspase-1 activity in epidural-related maternal fever: a single-centre, observational, mechanistic cohort study. Br J Anaesth. 2019;122:92–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

