Intermittent fasting reduces alpha-synuclein pathology and functional decline in a mouse model of Parkinson's disease
- PMID: 40368903
- PMCID: PMC12078643
- DOI: 10.1038/s41467-025-59249-5
Intermittent fasting reduces alpha-synuclein pathology and functional decline in a mouse model of Parkinson's disease
Abstract
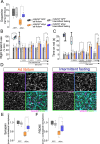
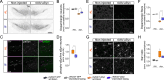
Parkinson's disease (PD) is a neurodegenerative disorder characterized by dopaminergic neuron degeneration and α-synuclein (aSyn) accumulation. Environmental factors play a significant role in PD progression, highlighting the potential of non-pharmacological interventions. This study investigates the therapeutic effects of intermittent fasting (IF) in an rAAV-aSyn mouse model of PD. IF, initiated four weeks post-induction of aSyn pathology, improved motor function and reduced dopaminergic neuron and axon terminal degeneration. Additionally, IF preserved dopamine levels and synaptic integrity in the striatum. Mechanistically, IF enhanced autophagic activity, promoting phosphorylated-aSyn clearance and reducing its accumulation in insoluble brain fractions. Transcriptome analysis revealed IF-induced modulation of inflammation-related genes and microglial activation. Validation in primary cultures confirmed that autophagy activation and inflammatory modulators (CCL17, IL-36RN) mitigate aSyn pathology. These findings suggest that IF exerts neuroprotective effects, supporting further exploration of IF and IF-mimicking therapies as potential PD treatments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

