Liposomal oncolytic adenovirus as a neoadjuvant therapy for triple-negative breast cancer
- PMID: 40368934
- PMCID: PMC12078467
- DOI: 10.1038/s41598-025-00211-2
Liposomal oncolytic adenovirus as a neoadjuvant therapy for triple-negative breast cancer
Abstract
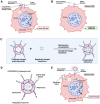
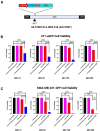
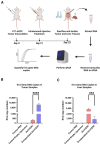
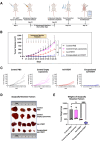
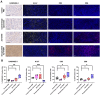
Breast cancer remains one of the leading causes of cancer-related death, with triple-negative breast cancer (TNBC) accounting for 15-20% of cases. TNBC, characterized by the absence of ER, PR, and HER2 protein, is an aggressive form of breast cancer that is unresponsive to hormonal therapies and HER2-targeted treatments, with fewer treatment options and poorer prognosis. Oncolytic adenoviruses (Ad) are a potential treatment option for TNBC but require coxsackievirus and adenovirus receptors (CAR) to effectively enter and transduce cancer cells. This study investigates a novel neoadjuvant therapy to improve the efficacy of an oncolytic Ad with human telomerase reverse transcriptase (Ad-hTERT) in CAR-low TNBC tumors using folate surface-modified liposomes to enhance delivery. This therapy helps deescalate treatment by reducing or eliminating the need for checkpoint inhibitors or toxic chemotherapy combinations. In vitro studies using CAR-low TNBC murine 4T1-eGFP cells, CAR-high TNBC human MDA-MB-231-GFP cells and several other TNBC human cancer cell lines with varying CAR expression demonstrated significantly higher cytotoxicity with encapsulated Ad-hTERT compared to Ad-hTERT. Similar results were observed in patient-derived primary TNBC cells. In vivo studies in immunocompetent mice with CAR-low 4T1-eGFP tumors revealed that encapsulated Ad-hTERT, administered as neoadjuvant therapy, resulted in stable or reduced tumor sizes, improved survival rates, higher apoptosis of cancer cells, lower cancer cell proliferation, and increased T-cell infiltration in resected tumors. Furthermore, encapsulated Ad-hTERT prevented lung metastasis and tumor recurrence at the primary site, resulting in higher survival rates in mice. Thus, liposomal encapsulation of Ad may be a viable strategy for treating TNBC.
Keywords: Coxsackievirus and adenovirus receptor; Liposomes; Metastasis; Neoadjuvant therapy; Oncolytic adenovirus; Triple-negative breast cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All tissues were collected by the Moores Cancer Center Biorepository from consented patients under a University of California, San Diego Human Research Protections Program Institutional Review Board approved protocol (HRPP# 181755). Biorepository subjects provide a written consent which is maintained in the Biorepository archives.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

