Dopaminergic action prediction errors serve as a value-free teaching signal
- PMID: 40369067
- PMCID: PMC12310545
- DOI: 10.1038/s41586-025-09008-9
Dopaminergic action prediction errors serve as a value-free teaching signal
Abstract
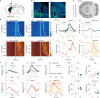
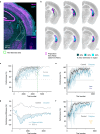
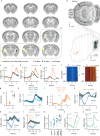
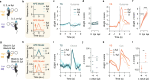
Choice behaviour of animals is characterized by two main tendencies: taking actions that led to rewards and repeating past actions1,2. Theory suggests that these strategies may be reinforced by different types of dopaminergic teaching signals: reward prediction error to reinforce value-based associations and movement-based action prediction errors to reinforce value-free repetitive associations3-6. Here we use an auditory discrimination task in mice to show that movement-related dopamine activity in the tail of the striatum encodes the hypothesized action prediction error signal. Causal manipulations reveal that this prediction error serves as a value-free teaching signal that supports learning by reinforcing repeated associations. Computational modelling and experiments demonstrate that action prediction errors alone cannot support reward-guided learning, but when paired with the reward prediction error circuitry they serve to consolidate stable sound-action associations in a value-free manner. Together we show that there are two types of dopaminergic prediction errors that work in tandem to support learning, each reinforcing different types of association in different striatal areas.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures













References
-
- Wood, W. & Runger, D. Psychology of habit. Annu. Rev. Psychol.67, 289–314 (2016). - PubMed
-
- Thorndike, E. L. Animal Intelligence: Experimental Studies (MacMillan, 1911).
-
- Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science275, 1593–1599 (1997). - PubMed
-
- Lindsey, J. & Litwin-Kumar, A. Action-modulated midbrain dopamine activity arises from distributed control policies. NeurIPS 2022 Conferencehttps://dl.acm.org/doi/10.5555/3600270.3600670 (2022). - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

