Fatty acid synthesis promotes mtDNA release via ETS1-mediated oligomerization of VDAC1 facilitating endothelial dysfunction in sepsis-induced lung injury
- PMID: 40369168
- PMCID: PMC12669790
- DOI: 10.1038/s41418-025-01524-5
Fatty acid synthesis promotes mtDNA release via ETS1-mediated oligomerization of VDAC1 facilitating endothelial dysfunction in sepsis-induced lung injury
Abstract
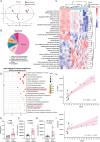
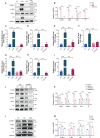
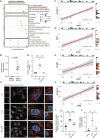
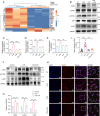
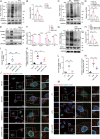
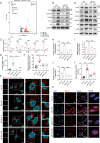
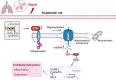
Sepsis involves endothelial cell dysfunction leading to the development of lung injury. Fatty acid synthesis contributes to the development of inflammatory injury in sepsis. However, the regulatory mechanisms of fatty acid synthesis-related endothelial activation remain unclear. In this study, we found that fatty acid synthesis in patients with sepsis was greatly disordered. Inhibition of fatty acid synthesis significantly alleviated sepsis-induced endothelial damage and lung injury both in vitro and in vivo. We further found that the release of mtDNA participated in fatty acid synthesis-related regulation of endothelial inflammatory and coagulation activation. Mechanistically, fatty acid synthesis promoted the oligomerization of voltage-dependent anion channel 1 (VDAC1) via ETS proto-oncogene 1 (ETS1)-mediated inhibition of VDAC1 ubiquitination, thereby leading to the increased release of mtDNA and subsequent activation of cGAS-STING signaling and pyroptosis in endothelial cells. Our findings revealed that fatty acid synthesis promoted endothelial dysfunction through mtDNA release, providing new insight into the therapeutic strategies for treating sepsis-associated lung injury.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: This research conformed to the ethics guidelines of the Declaration of Helsinki and was approved by the ethics committee of Ruijin Hospital. The animal protocols were approved by the Animal Ethics Committee of Shanghai Ruijin Hospital and were in line with the International Guidelines for Care and Use of Laboratory Animals (National Academy of Sciences Health Publication No. 85-23, revised in 1996).
Figures


References
-
- van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54:2450–64. - PubMed
-
- Wu F, Zhou X. The comparisons and limitations of Sepsis 2.0 and Sepsis 3.0. J Crit Care. 2018;47:350–1. - PubMed
-
- van Staa TP, Pate A, Martin GP, Sharma A, Dark P, Felton T et al. Sepsis and case fatality rates and associations with deprivation, ethnicity, and clinical characteristics: population-based case–control study with linked primary care and hospital data in England. Infection. 2024;52:1469–79. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

