Digitalized organoids: integrated pipeline for high-speed 3D analysis of organoid structures using multilevel segmentation and cellular topology
- PMID: 40369245
- PMCID: PMC12165853
- DOI: 10.1038/s41592-025-02685-4
Digitalized organoids: integrated pipeline for high-speed 3D analysis of organoid structures using multilevel segmentation and cellular topology
Abstract
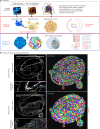
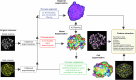
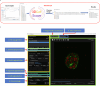
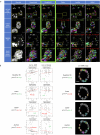
Organoids replicate tissue architecture and function and offer a unique opportunity to explore the impact of external perturbations in vitro. However, conducting large-scale screening procedures to investigate the effects of various stresses on cellular morphology and topology in these systems poses important challenges, including limitations in high-resolution three-dimensional (3D) imaging and accessible 3D analysis platforms. In this study, we introduce an AI-based multilevel segmentation and cellular topology pipeline for screening morphology and topology modifications in 3D cell culture at both the nuclear and cytoplasmic levels, as well as at the whole-organoid scale. We demonstrate the versatility of our approach through proof-of-concept experiments, encompassing well-characterized conditions and poorly explored mechanical stressors such as microgravity. By offering a user-friendly interface named 3DCellScope and a comprehensive set of tools for discovery-like assays in screening 3D organoid models, our pipeline demonstrates wide-ranging potential for applications in biomedical research.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.B., D.M. and G. Galisot are employed by QuantaCell. V.R. is the founder of QuantaCell that commercializes software and services partially based on the model used in this study, fully described in the Methods and accessible through the 3DCellScope.exe file. The other authors declare no competing interests.
Figures







References
-
- Stylianou, E. et al. A mathematical model for the role of cell accumulation in age-related intestinal tumorigenesis. PLoS ONE11, e0149562 (2016).
-
- Yin, J. et al. Mechanical forces contribute to aged-related joint degeneration. JCI Insight5, e132351 (2020).
-
- Drost, J. & Clevers, H. Organoids in cancer research. Nat. Rev. Cancer18, 407–418 (2018). - PubMed
-
- Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet.19, 671–687 (2018). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

