Small-molecule dissolution of stress granules by redox modulation benefits ALS models
- PMID: 40369342
- PMCID: PMC12463676
- DOI: 10.1038/s41589-025-01893-5
Small-molecule dissolution of stress granules by redox modulation benefits ALS models
Abstract
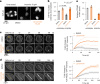
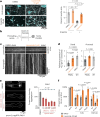
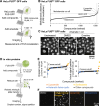
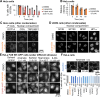
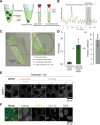
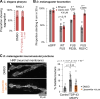
Neurodegenerative diseases, such as amyotrophic lateral sclerosis, are often associated with mutations in stress granule proteins. Aberrant stress granule condensate formation is associated with disease, making it a potential target for pharmacological intervention. Here, we identified lipoamide, a small molecule that specifically prevents cytoplasmic condensation of stress granule proteins. Thermal proteome profiling showed that lipoamide stabilizes intrinsically disordered domain-containing proteins, including SRSF1 and SFPQ, which are stress granule proteins necessary for lipoamide activity. SFPQ has redox-state-specific condensate dissolving behavior, which is modulated by the redox-active lipoamide dithiolane ring. In animals, lipoamide ameliorates aging-associated aggregation of a stress granule reporter protein, improves neuronal morphology and recovers motor defects caused by amyotrophic lateral sclerosis-associated FUS and TDP-43 mutants. Thus, lipoamide is a well-tolerated small-molecule modulator of stress granule condensation, and dissection of its molecular mechanism identified a cellular pathway for redox regulation of stress granule formation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.H. is the Scientific Founder of Dewpoint Therapeutics. A.H. and S.A. are Dewpoint Therapeutics shareholders. R.J.W. is a Scientific Advisor for Dewpoint Therapeutics. A.M.d.J.D. is an employee of Dewpoint Therapeutics, but his contribution to this work was before his employment. A.H., M.B. and R.J.W. filed a patent related to this work (US20200150107A1 and synchronized worldwide applications). Dewpoint Therapeutics contributed intellectually to this work in the SAR analysis of lipoamide analogs. All other experimental work either predates the foundation of Dewpoint Therapeutics or was performed independently. The other authors declare no competing interests.
Figures








References
-
- Robberecht, W. & Philips, T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci.14 248–264 (2013). - PubMed
-
- Miller, R. G., Mitchell, J. D. & Moore, D. H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev.2002, CD001447 (2012). - PubMed
-
- Abe, K. et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol.16, 505–512 (2017). - PubMed
-
- Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med.387, 1099–1110 (2022). - PubMed
-
- Deng, H., Gao, K. & Jankovic, J. The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol.10, 337–348 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- 103261/Z/13/Z/Wellcome Trust (Wellcome)
- R01 GM147677/GM/NIGMS NIH HHS/United States
- 390729961/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WT_/Wellcome Trust/United Kingdom
- R01GM147677/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

