Activity-driven myelin sheath growth is mediated by mGluR5
- PMID: 40369366
- PMCID: PMC12148935
- DOI: 10.1038/s41593-025-01956-9
Activity-driven myelin sheath growth is mediated by mGluR5
Abstract
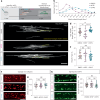
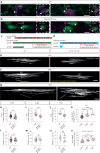
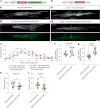
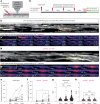
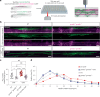
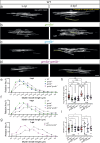
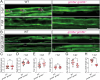
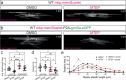
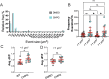
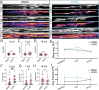
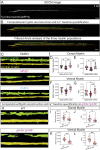
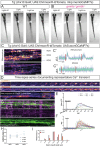
Myelination by oligodendrocytes in the central nervous system is influenced by neuronal activity, but the molecular mechanisms by which this occurs have remained unclear. Here we employed pharmacological, genetic, functional imaging and optogenetic-stimulation approaches in zebrafish to assess activity-regulated myelination in vivo. Pharmacological inhibition and activation of metabotropic glutamate receptor 5 (mGluR5) impaired and promoted myelin sheath elongation, respectively, during development, without otherwise affecting the oligodendrocyte lineage. Correspondingly, mGluR5 loss-of-function mutants exhibit impaired myelin growth, while oligodendrocyte-specific mGluR5 gain of function promoted sheath elongation. Functional imaging and optogenetic-stimulation studies revealed that mGluR5 mediates activity-driven high-amplitude Ca2+ transients in myelin. Furthermore, we found that long-term stimulation of neuronal activity drives myelin sheath elongation in an mGluR5-dependent manner. Together these data identify mGluR5 as a mediator of the influence of neuronal activity on myelination by oligodendrocytes in vivo, opening up opportunities to assess the functional relevance of activity-regulated myelination.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- BB/X009394/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- 108906/Z/15/Z/Wellcome Trust (Wellcome)
- R01 MH110504/MH/NIMH NIH HHS/United States
- 214244/Z/18/Z/Wellcome Trust (Wellcome)
- 214244/WT_/Wellcome Trust/United Kingdom
- H2020-MSCA-ITN-2018 813457/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- R01MH110504/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- U19 NS123719/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

