Dysregulated inflammation, oxidative stress, and protein quality control in diabetic HFpEF: unraveling mechanisms and therapeutic targets
- PMID: 40369521
- PMCID: PMC12080046
- DOI: 10.1186/s12933-025-02734-4
Dysregulated inflammation, oxidative stress, and protein quality control in diabetic HFpEF: unraveling mechanisms and therapeutic targets
Abstract
Background: Type 2 diabetes mellitus (T2DM) represents a significant risk factor for cardiovascular disease, particularly heart failure with preserved ejection fraction (HFpEF). HFpEF predominantly affects elderly individuals and women, and is characterized by dysfunctions associated with metabolic, inflammatory, and oxidative stress pathways. Despite HFpEF being the most prevalent heart failure phenotype in patients with T2DM, its underlying pathophysiological mechanisms remain inadequately elucidated.
Objective: This study aims to investigate the effects of diabetes mellitus on myocardial inflammation, oxidative stress, and protein quality control (PQC) mechanisms in HFpEF, with particular emphasis on insulin signaling, autophagy, and chaperone-mediated stress responses.
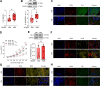
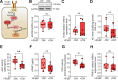
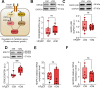
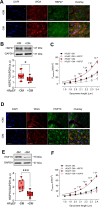
Methods: We conducted an analysis of left ventricular myocardial tissue from HFpEF patients, both with and without diabetes, employing a range of molecular, biochemical, and functional assays. The passive stiffness of cardiomyocytes (Fpassive) was assessed in demembranated cardiomyocytes before and after implementing treatments aimed at reducing inflammation (IL-6 inhibition), oxidative stress (Mito-TEMPO), and enhancing PQC (HSP27, HSP70). Inflammatory markers (NF-κB, IL-6, TNF-α, ICAM-1, VCAM-1, NLRP3), oxidative stress markers (ROS, GSH/GSSG ratio, lipid peroxidation), and components of signaling pathways (PI3K/AKT/mTOR, AMPK, MAPK, and PKG) were evaluated using western blotting, immunofluorescence, and ELISA techniques.
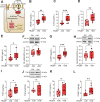
Results: Hearts from diabetic HFpEF patients exhibited significantly heightened inflammation, characterized by the upregulation of NF-κB, IL-6, and the NLRP3 inflammasome. This increase in inflammation was accompanied by elevated oxidative stress, diminished nitric oxide (NO) bioavailability, and impaired activation of the NO-sGC-cGMP-PKG signaling pathway. Notably, dysregulation of insulin signaling was observed, as indicated by decreased AKT phosphorylation and impaired autophagy regulation mediated by AMPK and mTOR. Additionally, PQC dysfunction was evidenced by reduced expression levels of HSP27 and HSP70, which correlated with increased cardiomyocyte passive stiffness. Targeted therapeutic interventions effectively reduced Fpassive, with IL-6 inhibition, Mito-TEMPO, and HSP administration leading to improvements in cardiomyocyte mechanical properties.
Conclusion: The findings of this study elucidate a mechanistic relationship among diabetes, inflammation, oxidative stress, and PQC impairment in the context of HFpEF. Therapeutic strategies that target these dysregulated pathways, including IL-6 inhibition, mitochondrial antioxidants, and chaperone-mediated protection, may enhance myocardial function in HFpEF patients with T2DM. Addressing these molecular dysfunctions could facilitate the development of novel interventions specifically tailored to the diabetic HFpEF population.
Keywords: Autophagy; Cardiomyocyte stiffness; Heart failure with preserved ejection fraction; Heat shock proteins; Inflammation; Insulin resistance; Oxidative stress; Protein quality control; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All samples were obtained with informed consent and approval from the local Ethics Committee. All procedures were performed according to the Declaration of Helsinki and were approved by the local ethics committee. Biopsies were obtained for the primary purpose of diagnosis following Ethics Committee approval numbers WO18.026, 20-6976 BR, 20-6976-1- BR and informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- IDF Diabetes Atlas, 9th edn. Federation ID, editor. Brussels: International Diabetes Federation (2019). 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

