2024 International Academy of Toxicologic Pathology (IATP) Satellite Symposium: New Approach Methodologies (NAMs) for Neurotoxicity Assessment and Regulatory Perspectives
- PMID: 40370040
- PMCID: PMC12349877
- DOI: 10.1177/01926233251335719
2024 International Academy of Toxicologic Pathology (IATP) Satellite Symposium: New Approach Methodologies (NAMs) for Neurotoxicity Assessment and Regulatory Perspectives
Abstract
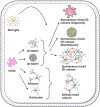
The International Academy of Toxicologic Pathology (IATP) Satellite Symposium on "New Approach Methodologies (NAMs) for Neurotoxicity Assessment and Regulatory Perspectives," organized in Spain, addressed the growing need for improved assessment of neurotoxicity. Traditional neurotoxicity assessment using in vivo animal studies are impractical for testing the substantial number of environmental chemicals that currently lack data and in the early detection of neuro-related adverse reactions in drug discovery. The NAMs, including human in vitro assays and small model organisms, have been developed for faster and cost-effective assessment of neurotoxic potential. While NAMs offer improved practicality, utility, and valuable mechanistic insights, their integration into regulatory decision-making requires robust scientific validation and technical characterization. Confidence in and regulatory application of NAMs data can be supported by mapping cellular outcomes to neuropathological findings in mammals, including humans, through the Adverse Outcome Pathway (AOP) framework, and the Integrated Approach to Testing and Assessment (IATA). Case studies presented demonstrated the application of NAMs in chemical and drug safety evaluations, focusing on developmental neurotoxicity (DNT), Parkinson's disease, and drug-induced seizures. In conjunction with in vivo toxicology studies, NAMs represent a significant step toward advancing chemical and drug toxicity assessment via hazard identification and drug screening safety assessments.
Keywords: Parkinson’s disease; adult neurotoxicity; adverse outcome pathway.; developmental neurotoxicity; new approach methodologies; seizure.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Professor EF is shareholders of DNTOX GmbH, offering neurotoxicity testing services. The other authors declared no real, perceived, or potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





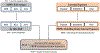
References
-
- Validation, Qualification, and Regulatory Acceptance of New Approach Methodologies (NIEHS) (2024). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

