Design and Applications of Polymersomes for Oral Drug Administration
- PMID: 40370090
- PMCID: PMC12123574
- DOI: 10.1021/acsami.5c04658
Design and Applications of Polymersomes for Oral Drug Administration
Abstract
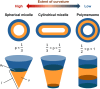
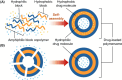
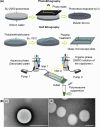
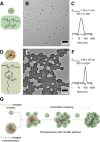
Polymersomes are nanostructures consisting of a hollow aqueous compartment enclosed by a coating of amphiphilic block copolymers. Owing to the entangled nature of their membrane, polymersomes exhibit higher mechanical stability than some other extensively studied nanostructures such as liposomes. This also enables the properties of the polymersome membrane to be more easily tuned to meet practical needs, making polymersomes promising carriers for drug delivery. Since the turn of the last century, the use of polymersomes has been exploited in diverse areas, ranging from protein therapy to medical imaging. Yet, discussions exploring the opportunities and challenges of the development of polymersomes for oral drug administration have been scant. This review addresses this gap by offering a snapshot of the current advances in the design, fabrication, and use of polymersomes as oral drug carriers. It is hoped that this review will not only highlight the practical potential of polymersomes for oral drug administration but will also shed light on the challenges determining the wider clinical potential of polymersomes in the forthcoming decades.
Keywords: amphiphilicity; block copolymers; colloidal behavior; controlled release; drug delivery; oral administration; polymersomes.
Figures




References
-
- Alibolandi M., Ramezani M., Abnous K., Sadeghi F., Hadizadeh F.. Comparative Evaluation of Polymersome Versus Micelle Structures as Vehicles for the Controlled Release of Drugs. J. Nanoparticle Res. 2015;17(2):76. doi: 10.1007/s11051-015-2878-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

