Incorporating graphene-modified mica and conductive nickel particles for enhanced corrosion resistance in epoxy zinc-rich coatings
- PMID: 40370413
- PMCID: PMC12075140
- DOI: 10.3389/fchem.2025.1544762
Incorporating graphene-modified mica and conductive nickel particles for enhanced corrosion resistance in epoxy zinc-rich coatings
Abstract
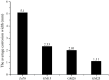
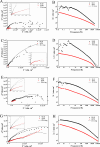
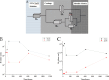
Epoxy zinc-rich coatings usually require high zinc content to ensure its anti-corrosion performance. However, excessive zinc powder content will reduce the mechanical properties of the coating, increase the economic cost, harm the environment, etc. Therefore, this paper aims to reduce the amount of zinc powder and improve the corrosion performance of epoxy zinc-rich coatings by introducing two kinds of conductive particle materials, conductive graphene-mica powder and conductive nickel. Conductive graphene was first loaded on mica powder and the obtained conductive graphene-mica powder and the conductive nickel were introduced to the epoxy zinc-rich coatings to partially replace zinc component. The anti-corrosion properties of the coating were systematically evaluated by EIS and salt spray test. The resulting epoxy zinc-rich coating with nickel powder or conductive graphene-mica demonstrates outstanding salt spray resistance, lasting up to 2,000 h, exhibiting superior anti-corrosion performance at reduced zinc content of 60% or 45% compared to conventional coatings with 70% pure zinc powder. This study introduces a novel conductive mica material and investigates conductive metal nickel additive, effectively reducing zinc content in epoxy zinc-rich coatings, which offers valuable insights for developing high-performance anti-corrosion coatings.
Keywords: anti-corrosion coatings; conductive mica; conductive nickel; epoxy zinc-rich coatings; graphene.
Copyright © 2025 Jiang, Zhang, Zhang, Shao, Zhu and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Study on the Corrosion Resistance of Graphene Oxide-Based Epoxy Zinc-Rich Coatings.Polymers (Basel). 2021 May 19;13(10):1657. doi: 10.3390/polym13101657. Polymers (Basel). 2021. PMID: 34069742 Free PMC article.
-
Improving the Corrosion Resistance of Zn-Rich Epoxy Coating with Three-Dimensional Porous Graphene.Polymers (Basel). 2023 Nov 1;15(21):4302. doi: 10.3390/polym15214302. Polymers (Basel). 2023. PMID: 37959980 Free PMC article.
-
Nacre-mimetic Strategy to Fabricate Waterborne FrGO/Zn/Epoxy Coatings with Dual Corrosion Protection.ACS Appl Mater Interfaces. 2023 Jun 14;15(23):28570-28580. doi: 10.1021/acsami.3c04423. Epub 2023 Jun 2. ACS Appl Mater Interfaces. 2023. PMID: 37265041
-
Correlations between the anti-corrosion properties and the photocatalytic behavior of epoxy coatings incorporating modified graphene oxide deposited on a zinc substrate.RSC Adv. 2024 Apr 3;14(16):10826-10841. doi: 10.1039/d4ra00413b. eCollection 2024 Apr 3. RSC Adv. 2024. PMID: 38577435 Free PMC article.
-
Insights into the Development of Corrosion Protection Coatings.Polymers (Basel). 2025 Jun 2;17(11):1548. doi: 10.3390/polym17111548. Polymers (Basel). 2025. PMID: 40508791 Free PMC article. Review.
References
-
- Al-Nafai I., Rzeszutek K., Lyon S., Jones C., Beaumont D. (2025). How aluminium additions improve the performance of zinc‐rich organic coatings. Mater. Corros. 76, 53–70. 10.1002/maco.202414529 - DOI
-
- Bai C.-Y., Lee J. L., Wen T. M., Hou K. H., Wu M. S., Ger M. D. (2011). The characteristics of chromized 1020 steel with electrical discharge machining and Ni electroplating pretreatments. Appl. Surf. Sci. 257 (8), 3529–3537. 10.1016/j.apsusc.2010.11.070 - DOI
-
- Brug G. J., van den Eeden A. L. G., Sluyters-Rehbach M., Sluyters J. (1984). The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 176, 275–295. 10.1016/0368-1874(84)83477-2 - DOI
-
- Chao Z., Chao W., Bo J., Renguo S. (2023). Preparation and research of AZ31 magnesium alloy micro-arc oxidation/epoxy rensin-nickel powder conductive coating. Mater. Prot. 56 (8), 64–69.
-
- Chen Z., Cai Y., Lu Y., Cao Q., Lv P., Zhang Y., et al. (2022). Preparation and performance study of carboxy-functionalized graphene oxide composite polyaniline modified water-based epoxy zinc-rich coatings. Coatings 12 (6), 824. 10.3390/coatings12060824 - DOI
LinkOut - more resources
Full Text Sources

