Acute-phase proteins as biomarkers of inflammation in HIV patients with latent tuberculosis: a prospective study
- PMID: 40370442
- PMCID: PMC12075211
- DOI: 10.3389/fimmu.2025.1551775
Acute-phase proteins as biomarkers of inflammation in HIV patients with latent tuberculosis: a prospective study
Abstract
Introduction: Tuberculosis (TB) remains the primary cause of death among individuals infected with HIV, increasing the risk of contracting TB by up to 26 times. This co-infection complicates the diagnosis and treatment of TB, ultimately affecting outcomes adversely. Acute-phase proteins (APPs), markers of inflammation, are significantly elevated during infections and serve as critical indicators of inflammation resulting from infectious diseases.
Methods: In this prospective study, HIV-positive individuals at antiretroviral therapy (ART) clinics were screened for latent tuberculosis infection (LTBI) before starting isoniazid (INH) prophylaxis. Initially, 101 patients were enrolled, with 71 completing a six-month follow-up on INH prophylaxis. LTBI was diagnosed using QuantiFERON-TB Gold plus, categorizing participants as HIV-positive with LTBI (n=30) and HIV-positive without LTBI (n=71).
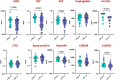
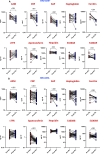
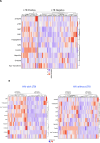
Results: Plasma levels of alpha-2-macroglobulin (A2M), C-reactive protein (CRP), serum amyloid P (SAP), haptoglobin, ferritin, soluble transferrin receptor (sTFR), apotransferrin, hepcidin, and S100A8/A9 were assessed using multiplex and quantikine assays.At baseline, levels of A2M, CRP, SAP, ferritin, hepcidin, and S100A9 were significantly elevated in HIV-positive patients with LTBI compared to those without LTBI (A2M, p=0.005; CRP, p<0.001; SAP, p=0.0006; ferritin, p<0.001; hepcidin, p=0.001; S100A9, p=0.001). Following six months of INH prophylaxis, significant reductions in these markers were observed in both groups, suggesting a reduction in inflammation.
Discussion: Our findings indicate that a baseline profile of APPs can effectively reflect the inflammatory status of HIV patients with LTBI. These inflammatory markers tend to decrease following effective INH treatment, underscoring their potential utility in monitoring disease progression and treatment response in this vulnerable population.
Keywords: HIV; LTBI; acute-phase proteins; biomarkers; isoniazid preventive treatment (IPT).
Copyright © 2025 Pavan Kumar, M, Nancy, Kumar, M, Ahamed, Kumar, S, S, R, Baskaran, Hissar and Babu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

