Therapeutic targeting of alternative pathway and C5 but not C5a protects from disease development in a preclinical model of autoimmune blistering dermatosis
- PMID: 40370446
- PMCID: PMC12076022
- DOI: 10.3389/fimmu.2025.1560468
Therapeutic targeting of alternative pathway and C5 but not C5a protects from disease development in a preclinical model of autoimmune blistering dermatosis
Abstract
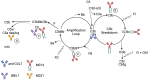
Introduction: Epidermolysis Bullosa Acquisita (EBA) is an autoimmune blistering dermatosis characterized by autoantibodies (AAbs) against type VII collagen (COL7) located at the dermal epidermal junction (DEJ). Local complement activation drives C5a generation associated with neutrophil recruitment and activation resulting in skin lesions and inflammation. Here we tested the impact of C5a/C5adesArg, C5 or combined C5 and alternative pathway (AP) targeting on disease development and skin inflammation in a preclinical mouse model mimicking the effector phase of EBA.
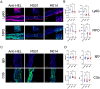
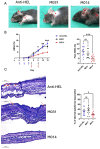
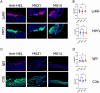
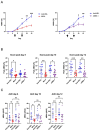
Methods: C57BL/6 mice were treated subcutaneously with purified rabbit anti-mouse-COL7 IgG in the presence of IgG1 mAbs directed against murine C5a/C5adesArg (M031), C5 (mBB5.1), a bifunctional protein comprising mBB5.1 fused to an active fragment of the AP inhibitor factor H (M014) or an IgG1 isotype control mAb. Formation of skin lesions was evaluated 12 days every other day. On day 12, DEJ separation, IgG AAb and C3b deposition and neutrophil infiltration was assessed.
Results: Isotype IgG1-treated mice developed first skin lesions on day 4 peaking on day 12. Prophylactic treatment with either M031 or M014 markedly reduced the development of skin lesions, the dermal/epidermal separation and neutrophil recruitment. Surprisingly, C5 or combined AP/C5 inhibition by M014 but not C5a/C5adesArg-targeting by M031 reduced the development of skin lesions and dermal/epidermal separation in the setting of therapeutic treatment. IgG and C3b deposition was not affected by either treatment. Importantly, direct comparison of isolated C5 targeting by mBB5.1 vs. combined AP/C5 inhibition by M014 revealed that M014 reduced the development of skin lesions earlier and more pronounced than mBB5.1.
Discussion: Our findings identify combined C5/AP targeting as a novel therapeutic option for autoimmune blistering dermatoses.
Keywords: C5a; EBA; alternative pathway; bullous pemphigoid; complement.
Copyright © 2025 Laffer, Ohms, Kenno, Tsui, Ehlers-Jeske, Song, Song and Köhl.
Conflict of interest statement
Authors PT and WS were employed by the company Kira Pharmaceuticals. W-CS has equity interest in, and is an inventor of patents licensed to, Kira Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Kira Pharmaceuticals. The funder was involved in the study design, review and editing of the manuscript.
Figures

References
-
- Sitaru C, Kromminga A, Hashimoto T, Brocker EB, Zillikens D. Autoantibodies to type VII collagen mediate Fcgamma-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Am J Pathol. (2002) 161:301–11. doi: 10.1016/s0002-9440(10)64182-x - DOI - PMC - PubMed
-
- Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, et al. . Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. (2004) 204:519–27. doi: 10.1002/path.1674 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

