Synaptic plasticity facilitates oscillations in a V1 cortical column model with multiple interneuron types
- PMID: 40370493
- PMCID: PMC12075120
- DOI: 10.3389/fncom.2025.1568143
Synaptic plasticity facilitates oscillations in a V1 cortical column model with multiple interneuron types
Abstract
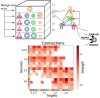
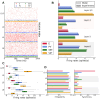
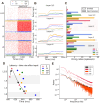
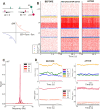
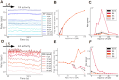
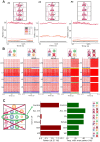
Neural rhythms are ubiquitous in cortical recordings, but it is unclear whether they emerge due to the basic structure of cortical microcircuits or depend on function. Using detailed electrophysiological and anatomical data of mouse V1, we explored this question by building a spiking network model of a cortical column incorporating pyramidal cells, PV, SST, and VIP inhibitory interneurons, and dynamics for AMPA, GABA, and NMDA receptors. The resulting model matched in vivo cell-type-specific firing rates for spontaneous and stimulus-evoked conditions in mice, although rhythmic activity was absent. Upon introduction of long-term synaptic plasticity in the form of an STDP rule, broad-band (15-60 Hz) oscillations emerged, with feedforward/feedback input streams enhancing/suppressing the oscillatory drive, respectively. These plasticity-triggered rhythms relied on all cell types, and specific experience-dependent connectivity patterns were required to generate oscillations. Our results suggest that neural rhythms are not necessarily intrinsic properties of cortical circuits, but rather they may arise from structural changes elicited by learning-related mechanisms.
Keywords: cortical column; interneurons; oscillations; synaptic plasticity; visual cortex.
Copyright © 2025 Moreni, Zou, Pennartz and Mejias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Cell-type-specific firing patterns in a V1 cortical column model depend on feedforward and feedback-driven states.PLoS Comput Biol. 2025 Apr 23;21(4):e1012036. doi: 10.1371/journal.pcbi.1012036. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40267074 Free PMC article.
-
Flexible Frequency Switching in Adult Mouse Visual Cortex Is Mediated by Competition Between Parvalbumin and Somatostatin Expressing Interneurons.Neural Comput. 2021 Mar 26;33(4):926-966. doi: 10.1162/neco_a_01369. Neural Comput. 2021. PMID: 33513330
-
Activation of Somatostatin Interneurons by Nicotinic Modulator Lypd6 Enhances Plasticity and Functional Recovery in the Adult Mouse Visual Cortex.J Neurosci. 2020 Jul 1;40(27):5214-5227. doi: 10.1523/JNEUROSCI.1373-19.2020. Epub 2020 May 28. J Neurosci. 2020. PMID: 32467358 Free PMC article.
-
Portrait of visual cortical circuits for generating neural oscillation dynamics.Cogn Neurodyn. 2021 Feb;15(1):3-16. doi: 10.1007/s11571-020-09623-4. Epub 2020 Aug 10. Cogn Neurodyn. 2021. PMID: 34109010 Free PMC article. Review.
-
Cortical interneurons: fit for function and fit to function? Evidence from development and evolution.Front Neural Circuits. 2023 May 4;17:1172464. doi: 10.3389/fncir.2023.1172464. eCollection 2023. Front Neural Circuits. 2023. PMID: 37215503 Free PMC article. Review.
Cited by
-
Adaptive arousal regulation: Pharmacologically shifting the peak of the Yerkes-Dodson curve by catecholaminergic enhancement of arousal.Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2419733122. doi: 10.1073/pnas.2419733122. Epub 2025 Jul 7. Proc Natl Acad Sci U S A. 2025. PMID: 40623185
References
LinkOut - more resources
Full Text Sources

