Novel carbazole attenuates vascular remodeling through STAT3/CIAPIN1 signaling in vascular smooth muscle cells
- PMID: 40370537
- PMCID: PMC12069901
- DOI: 10.1016/j.apsb.2024.12.035
Novel carbazole attenuates vascular remodeling through STAT3/CIAPIN1 signaling in vascular smooth muscle cells
Abstract
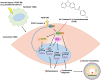
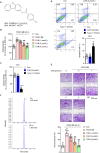
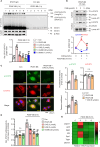
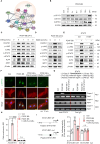
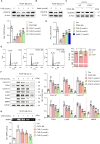
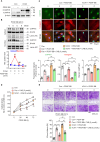
This study investigated the molecular mechanism of phenotypic switching of vascular smooth muscle cells (VSMCs), which play a crucial role in vascular remodeling using 9H-Carbazol-3-yl 4-aminobenzoate (CAB). CAB significantly attenuated platelet-derived growth factor (PDGF)-induced VSMC proliferation and migration. CAB suppressed PDGF-induced STAT3 activation by directly binding to the SH2 domain of STAT3. Downregulation of STAT3 phosphorylation by CAB attenuated CIAPIN1/JAK2/STAT3 axis through a decrease in CIAPIN1 transcription. Furthermore, abrogated CIAPIN1 decreased KLF4-mediated VSMC dedifferentiation and increased CDKN1B-induced cell cycle arrest and MMP9 suppression. CAB inhibited intimal hyperplasia in injury-induced neointima animal models by inhibition of the CIAPIN1/JAK2/STAT3 axis. However, CIAPIN1 overexpression attenuated CAB-mediated suppression of VSMC proliferation, migration, phenotypic switching, and intimal hyperplasia. Our study clarified the molecular mechanism underlying STAT3 inhibition of VSMC phenotypic switching and vascular remodeling and identified novel active CAB. These findings demonstrated that STAT3 can be a major regulator to control CIAPIN1/JAK2/STAT3 axis that may be a therapeutic target for treating vascular proliferative diseases.
Keywords: Atherosclerosis; Carbazole; Cytokine induced apoptosis inhibitor 1; Janus tyrosine kinase 2; Krüppel-like factor 4; Phenotyping switching; Signal transducer and activator of transcription 3; Vascular smooth muscle cell.
© 2025 The Authors.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Basatemur G.L., Jorgensen H.F., Clarke M.C.H., Bennett M.R., Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 2019;16:727–744. - PubMed
-
- Petsophonsakul P., Furmanik M., Forsythe R., Dweck M., Schurink G.W., Natour E., et al. Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2019;39:1351–1368. - PubMed
-
- Lacolley P., Regnault V., Nicoletti A., Li Z., Michel J.B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

