Long-chain acylcarnitine deficiency promotes hepatocarcinogenesis
- PMID: 40370557
- PMCID: PMC12069247
- DOI: 10.1016/j.apsb.2025.01.017
Long-chain acylcarnitine deficiency promotes hepatocarcinogenesis
Abstract
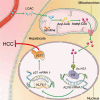
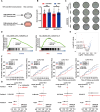
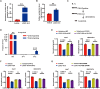
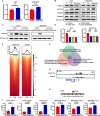
Despite therapy with potent antiviral agents, chronic hepatitis B (CHB) patients remain at high risk of hepatocellular carcinoma (HCC). While metabolites have been rediscovered as active drivers of biological processes including carcinogenesis, the specific metabolites modulating HCC risk in CHB patients are largely unknown. Here, we demonstrate that baseline plasma from CHB patients who later developed HCC during follow-up exhibits growth-promoting properties in a case-control design nested within a large-scale, prospective cohort. Metabolomics analysis reveals a reduction in long-chain acylcarnitines (LCACs) in the baseline plasma of patients with HCC development. LCACs preferentially inhibit the proliferation of HCC cells in vitro at a physiological concentration and prevent the occurrence of HCC in vivo without hepatorenal toxicity. Uptake and metabolism of circulating LCACs increase the intracellular level of acetyl coenzyme A, which upregulates histone H3 Lys14 acetylation at the promoter region of KLF6 gene and thereby activates KLF6/p21 pathway. Indeed, blocking LCAC metabolism attenuates the difference in KLF6/p21 expression induced by baseline plasma of HCC/non-HCC patients. The deficiency of circulating LCACs represents a driver of HCC in CHB patients with viral control. These insights provide a promising direction for developing therapeutic strategies to reduce HCC risk further in the antiviral era.
Keywords: Acetyl coenzyme A; CUT&Tag; Chemoprevention; H3K14; Hepatocellular carcinoma; KLF6; Long-chain acylcarnitine; Metabolomics.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. - PubMed
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Liu Z., Jiang Y., Yuan H., Fang Q., Cai N., Suo C., et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70:674–683. - PubMed
-
- Liaw Y.F., Sung J.J., Chow W.C., Farrell G., Lee C.Z., Yuen H., et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. - PubMed
LinkOut - more resources
Full Text Sources

