COVID-19 and blood group-related antigens: can natural anti-carbohydrate antibodies provide innate protection from symptomatic SARS-CoV-2 infection?
- PMID: 40370735
- PMCID: PMC12074924
- DOI: 10.3389/fmed.2025.1554785
COVID-19 and blood group-related antigens: can natural anti-carbohydrate antibodies provide innate protection from symptomatic SARS-CoV-2 infection?
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily targets respiratory mucosa, causing coronavirus disease 2019 (COVID-19). Susceptibility and severity of COVID-19 may be influenced by predisposing factors including blood groups. In this study, we investigated whether natural anti-carbohydrate antibodies provide innate protection against SARS-CoV-2 and influence disease severity.
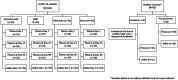
Methodology: We used samples (plasma and saliva) from a longitudinal cohort study in Bangladesh that enrolled 100 COVID-19 symptomatic and asymptomatic patients. We also enrolled 21 and 38 healthy controls during the pandemic period and pre-pandemic period, respectively. We phenotype ABO blood grouping from blood and determined Lewis and secretor status (H antigen) from the saliva samples. We quantified natural anti-carbohydrate antibodies (anti-A, anti-B, anti-Tn-Mono and anti-αGal IgG, IgA, and IgM) from plasma collected at enrollment. We also explored the trend of natural anti-carbohydrate antibodies until 3 months of convalescence period among the COVID-19 patients (day 14 and day 90 from enrollment). Antibody quantification and ABH/Lewis phenotyping were performed using enzyme-linked immunosorbent assay (ELISA).
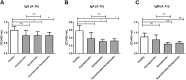
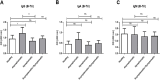
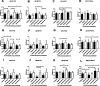
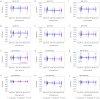
Results: We included 99 COVID-19 patients and 59 healthy controls assessing the differences of natural antibody titer during enrollment, while 95 patients were analyzed exploring Lewis and secretor status with natural antibody titer and disease status. We did not find significant difference in the distribution for neither ABO blood groups nor non-secretors and Lewis-negative individuals among asymptomatic or symptomatic patients and healthy controls. Nonetheless, we observed lower anti-A antibody titers among symptomatic patients compared to healthy controls. We also identified slight differences in antibody titers linked to age and gender. Anti-A and anti-B antibodies among asymptomatic patients had a higher trend up to 3 months from infection compared to symptomatic patients.
Conclusion: Higher natural anti-A and anti-B antibody titers may offer protection against symptomatic COVID-19 infections. Gender and blood group differences indicate potential innate immune factors influencing disease severity, but larger studies are needed to confirm these findings.
Keywords: Bangladesh; COVID-19; HBGA; Lewis status; SARS-CoV-2; blood group; natural anti-carbohydrate antibodies; secretor status.
Copyright © 2025 Ahmed, Breiman, Akhtar, Babu, Pervin, Firoj, Akter, Qadri, Chowdhury, Bhuiyan, Le Pendu and Ruvoën-Clouet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akter A, Ahmed T, Tauheed I, Akhtar M, Rahman SIA, Khaton F, et al. Disease characteristics and serological responses in patients with differing severity of COVID-19 infection: a longitudinal cohort study in Dhaka, Bangladesh. PLoS Negl Trop Dis. (2022) 16:e0010102. doi: 10.1371/journal.pntd.0010102, PMID: - DOI - PMC - PubMed
-
- Matzhold EM, Berghold A, Bemelmans MKB, Banfi C, Stelzl E, Kessler HH, et al. Lewis and ABO histo-blood types and the secretor status of patients hospitalized with COVID-19 implicate a role for ABO antibodies in susceptibility to infection with SARS-CoV-2. Transfusion. (2021) 61:2736–45. doi: 10.1111/trf.16567, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

