Micellization behavior of an imidazolium surface-active ionic liquid within aqueous solutions of deep eutectic solvents: a comparative spectroscopic study
- PMID: 40370844
- PMCID: PMC12076072
- DOI: 10.1039/d5ra01940k
Micellization behavior of an imidazolium surface-active ionic liquid within aqueous solutions of deep eutectic solvents: a comparative spectroscopic study
Abstract
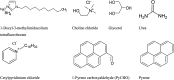
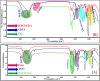
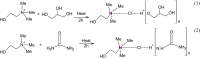
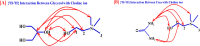
Ionic liquids and deep eutectic solvents are topics of immense importance and are attracting many researchers worldwide owing to their green nature and broader application potential. These green solvents have been widely used in synthesis, catalysis and biocatalysis, nanoscience, pharmaceutics, etc. Hence, it is exciting to see how ionic liquids behave within deep eutectic solvents. Here, we examined the aggregation behavior of an imidazolium-based surface-active ionic liquid (SAIL), 1-decyl-3-methylimidazolium tetrafluoroborate [Dmim][BF4], within aqueous solution of 5 wt% DES choline-based deep eutectic solvents (DESs). Two choline-based DESs (ChCl-urea and ChCl-Gly) were prepared by heating a mixture of 1 : 2 molar ratios of an ammonium salt (choline chloride) with hydrogen bond donors (urea and glycerol). The DESs were characterized using FTIR and 1H-NMR spectroscopic techniques. Micellization behavior of the SAIL [Dmim][BF4] within these aqueous DESs media was investigated using fluorescence, FTIR, dynamic light scattering (DLS), 1H-NMR, and NOESY. The information about the local microenvironment surrounding the probe molecules and size of the aggregates of [Dmim][BF4] in the presence of 5 wt% of aqueous DESs were obtained from fluorescence and DLS, respectively. DLS results showed that IL [Dmim][BF4] forms relatively larger micelles within aqueous solutions of DES ChCl-urea (avg. hydrodynamic radii = 94.6 nm) compared with ChCl-Gly (avg. hydrodynamic radii = 82.8 nm). A significant decrease in the critical micellar concentration and an increase in aggregation number (N agg) were observed, clearly indicating that micellization of IL [Dmim][BF4] is greatly favored in the DES solutions. FTIR study depicts the strength of intermolecular interactions such as hydrogen bonding, ion-ion pair interactions, and dipole-dipole interactions between the ILs and DESs. The 1H-NMR data showed that differences in chemical shifts can provide significant indication about the IL-DES interactions. 1H-NMR and 1H-1H 2D NOESY spectroscopy were employed to gain insights into these IL-DES interactions that are responsible for the aggregation behavior of the IL [Dmim][BF4] within aqueous DES solutions. It was observed that IL [Dmim][BF4] forms self-assembled structures within the aqueous DESs media. The current results are expected to be useful for colloidal aspects of ILs and DESs and their mixtures with water.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare no competing financial interest.
Figures






References
-
- Toledo Hijo A. A. C. Silva E. K. Meirelles A. A. D. Cunha R. L. Meirelles A. J. A. Novel naturally derived encapsulation agents in the ionic liquid form for sustainable emulsion-based products. Sustainable Food Technol. 2023;1:275–279.
-
- Gale E. Wirawan R. H. Silveira R. L. Pereira C. S. Johns M. A. Skaf M. S. Scott J. L. Directed discovery of greener cosolvents: new cosolvents for use in ionic liquid based organic electrolyte solutions for cellulose dissolution. ACS Sustainable Chem. Eng. 2016;4:6200–6207.
-
- Clark J. H. Farmer T. J. Davila L. H. Sherwood J. Circular economy design considerations for research and process development in the chemical sciences. Green Chem. 2016;18:3914–3934.
-
- Gilbertson L. M. Zimmerman J. B. Plata D. L. Hutchison J. E. Anastas P. T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015;44:5758–5777. - PubMed
-
- Egorova K. S. Gordeev E. G. Ananikov V. P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017;117:7132–7189. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

