Indomethacin-incorporated microemulsion-laden contact lenses for improved ocular drug delivery and therapeutic efficacy
- PMID: 40370851
- PMCID: PMC12077302
- DOI: 10.1039/d5ra01046b
Indomethacin-incorporated microemulsion-laden contact lenses for improved ocular drug delivery and therapeutic efficacy
Abstract
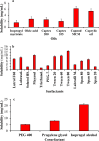
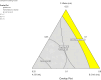
Conventional eye drops are associated with several limitations, including rapid drug clearance and low bioavailability, with only about 5% of the administered drug reaching the cornea to demonstrate therapeutic efficacy. Thus, to address these challenges, indomethacin (IND)-loaded microemulsion (Me)-embedded soft contact lenses (CLs) were developed to improve ocular drug delivery. The D-optimal mixture design was employed to optimize the composition of the Me formulation. Independent variables included Capmul MCM (oil phase), S mix (Tween 80/isopropyl alcohol), and water, while dependent variables were the globule size, transmittance (%), and drug release profile. The optimized Me exhibited a globule diameter of 45.69 ± 1.85 nm and a transmittance of 99.4% ± 1.59%. The globule dispersion index (PDI) was 0.33 ± 0.06, and the zeta potential (ZP) was 0.657 ± 0.012 mV. Soft CLs were developed using free radical polymerization and fortified with the Me loaded with the drug through direct loading and soaking techniques. Direct loading achieved a swelling percentage of 96.37% ± 1.8% and a transmittance of 96.5% ± 0.3%, while soaking resulted in 97.57% ± 1.4% swelling and 97.3% ± 1.3% transmittance. A drug content of 19.76 ± 0.23 μg per lenses for direct loading and 29.8 ± 0.2 μg per lenses for soaking demonstrated that the efficacy of the soaking technique was higher than that of direct loading. Moreover, drug release studies showed that the Me-laden lenses prepared using the direct loading technique released 44.00% ± 0.53% to 53.00% ± 0.59% of the drug within the first 6 h, while the lenses prepared via soaking released 62.58% ± 1.56% to 97.64% ± 1.52% of the drug within the first 6 h, followed by regulated drug release for up to 28 h, maintaining clarity and drug loading. Ocular irritancy test results indicated negligible irritation, suggesting that the Me-laden lenses are a safe and effective platform to manage ocular inflammation with the controlled delivery of IND.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Levofloxacin-loaded silicone contact lenses materials for ocular drug delivery.J Biomater Appl. 2025 Mar;39(8):855-865. doi: 10.1177/08853282241312089. Epub 2024 Dec 24. J Biomater Appl. 2025. PMID: 39719268
-
Bimatoprost-Loaded Silica Shell-Coated Nanoparticles-Laden Soft Contact Lenses to Manage Glaucoma: In Vitro and In Vivo Studies.AAPS PharmSciTech. 2021 Dec 23;23(1):33. doi: 10.1208/s12249-021-02199-0. AAPS PharmSciTech. 2021. Retraction in: AAPS PharmSciTech. 2022 May 31;23(5):155. doi: 10.1208/s12249-022-02306-9. PMID: 34950994 Retracted.
-
Development of brimonidine niosomes laden contact lenses for extended release and promising delivery system in glaucoma treatment.Daru. 2024 Jun;32(1):161-175. doi: 10.1007/s40199-023-00500-z. Epub 2023 Dec 29. Daru. 2024. PMID: 38158475 Free PMC article.
-
Advances and challenges in the nanoparticles-laden contact lenses for ocular drug delivery.Int J Pharm. 2021 Oct 25;608:121090. doi: 10.1016/j.ijpharm.2021.121090. Epub 2021 Sep 14. Int J Pharm. 2021. PMID: 34530102 Review.
-
A review on therapeutic contact lenses for ocular drug delivery.Drug Deliv. 2016 Oct;23(8):3017-3026. doi: 10.3109/10717544.2016.1138342. Epub 2016 Jan 29. Drug Deliv. 2016. PMID: 26821766 Review.
References
-
- Onugwu A. L. Nwagwu C. S. Onugwu O. S. Echezona A. C. Agbo C. P. Ihim S. A. Emeh P. Nnamani P. O. Attama A. A. Khutoryanskiy V. V. J. Controlled Release. 2023;354:465–488. - PubMed
-
- Popescu V. Soceanu A. Dobrinas S. Stanciu G. Ovidius Univ. Ann. Chem. 2012;23:87–91.
-
- Laddha U. D. Kshirsagar S. J. J. Drug Delivery Sci. Technol. 2021;61:102112.
-
- Dhaval M. Devani J. Parmar R. Soniwala M. M. Chavda J. J. Drug Delivery Sci. Technol. 2020;55:101373.
LinkOut - more resources
Full Text Sources

