Improving the Stability of Colloidal CsPbBr3 Nanocrystals with an Alkylphosphonium Bromide as Surface Ligand Pair
- PMID: 40370949
- PMCID: PMC12070459
- DOI: 10.1021/acsenergylett.5c00124
Improving the Stability of Colloidal CsPbBr3 Nanocrystals with an Alkylphosphonium Bromide as Surface Ligand Pair
Abstract
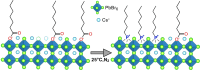
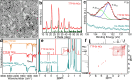
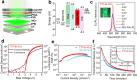
In this study, we synthesized a phosphonium-based ligand, trimethyl(tetradecyl)phosphonium bromide (TTP-Br), and employed it in the postsynthesis surface treatment of Cs-oleate-capped CsPbBr3 nanocrystals (NCs). The photoluminescence quantum yield (PLQY) of the NCs increased from ∼60% to more than 90% as a consequence of replacing Cs-oleate with TTP-Br ligand pairs. Density functional theory calculations revealed that TTP+ ions bind to the NC surface by occupying Cs+ surface sites and orienting one of their P-CH3 bonds perpendicular to the surface, akin to quaternary ammonium passivation. Importantly, TTP-Br-capped NCs exhibited higher stability in air compared to didodecyldimethylammonium bromide-capped CsPbBr3 NCs (which are considered a benchmark system), retaining ∼90% of their PLQY after 6 weeks of air exposure. Light-emitting diodes fabricated with TTP-Br-capped NCs achieved a maximum external quantum efficiency of 17.2%, demonstrating the potential of phosphonium-based molecules as surface ligands for CsPbBr3 NCs in optoelectronic applications.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Surface Chemistry of Lead Halide Perovskite Colloidal Nanocrystals.Acc Chem Res. 2023 Jul 4;56(13):1815-1825. doi: 10.1021/acs.accounts.3c00174. Epub 2023 Jun 22. Acc Chem Res. 2023. PMID: 37347953 Free PMC article.
-
Surface Crystal Growth of Perovskite Nanocrystals via Postsynthetic Lead(II) Bromide Treatment to Increase the Colloidal Stability and Efficiency of Light-Emitting Devices.ACS Appl Mater Interfaces. 2020 Oct 7;12(40):45574-45581. doi: 10.1021/acsami.0c13212. Epub 2020 Sep 24. ACS Appl Mater Interfaces. 2020. PMID: 32914951
-
Short-branched alkyl sulfobetaine-passivated CsPbBr3 nanocrystals for efficient green light emitting diodes.Nanoscale. 2024 Apr 18;16(15):7387-7395. doi: 10.1039/d4nr00965g. Nanoscale. 2024. PMID: 38545886
-
Partial Ligand Stripping from CsPbBr3 Nanocrystals Improves Their Performance in Light-Emitting Diodes.ACS Appl Mater Interfaces. 2024 Mar 6;16(9):11627-11636. doi: 10.1021/acsami.3c15201. Epub 2024 Feb 21. ACS Appl Mater Interfaces. 2024. PMID: 38381521 Free PMC article.
-
Blue-Emitting CsPbBr3 Nanocrystals: Synthesis Progress and Bright Photoluminescence.Langmuir. 2025 Mar 11;41(9):5762-5781. doi: 10.1021/acs.langmuir.4c05108. Epub 2025 Feb 26. Langmuir. 2025. PMID: 40008989 Review.
References
-
- Bodnarchuk M. I.; Boehme S. C.; Ten Brinck S.; Bernasconi C.; Shynkarenko Y.; Krieg F.; Widmer R.; Aeschlimann B.; Günther D.; Kovalenko M. V.; Infante I. Rationalizing and controlling the surface structure and electronic passivation of cesium lead halide nanocrystals. ACS Energy Letters 2019, 4 (1), 63–74. 10.1021/acsenergylett.8b01669. - DOI - PMC - PubMed
-
- Byranvand M. M.; Otero-Martínez C.; Ye J.; Zuo W.; Manna L.; Saliba M.; Hoye R. L.; Polavarapu L. Recent progress in mixed a-site cation halide perovskite thin-films and nanocrystals for solar cells and light-emitting diodes. Advanced Optical Materials 2022, 10 (14), 2200423.10.1002/adom.202200423. - DOI
-
- Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X= Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15 (6), 3692–3696. 10.1021/nl5048779. - DOI - PMC - PubMed
-
- Liu D.; Guo Y.; Que M.; Yin X.; Liu J.; Xie H.; Zhang C.; Que W. Metal halide perovskite nanocrystals: application in high-performance photodetectors. Materials Advances 2021, 2 (3), 856–879. 10.1039/D0MA00796J. - DOI
LinkOut - more resources
Full Text Sources
