Aberrant DNMT1-mediated DACH1 methylation is associated with colorectal adenoma-to-carcinoma progression
- PMID: 40370966
- PMCID: PMC12075005
- DOI: 10.3389/ebm.2025.10469
Aberrant DNMT1-mediated DACH1 methylation is associated with colorectal adenoma-to-carcinoma progression
Abstract
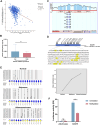
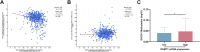
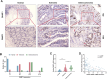
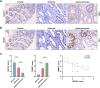
Colorectal cancer (CRC) remains a major contributor to cancer-related morbidity and mortality. While Dachshund homolog 1 (DACH1) was recognized as a critical regulator in cancer progression, its role in promoting or suppressing tumor development remains a subject of ongoing debate. This study aimed to elucidate the role of DACH1 in CRC progression and its underlying regulation mechanisms. The expression levels of Methyltransferase 1 (DNMT1) and DACH1, as well as its methylation status were assessed through a combination of TCGA data analysis and experimental validation using immunohistochemistry, PCR, methylation-specific PCR, and bisulfite sequencing RCR on 120 clinical samples, comprising normal mucosa, adenomas, and adenocarcinomas. The relationships among them were evaluated using Pearson or Spearman correlation analysis. The associations between the DACH1 and DNMT1 levels and clinicopathological parameters were examined to determine their clinical relevance. A progressive decrease in DACH1 expression and a concomitant increase in DACH1 promoter methylation and DNMT1 expression were observed from normal mucosa to adenoma and adenocarcinoma tissues. Higher DNMT1 expression and lower DACH1 expression were associated with poorer clinical outcomes, including worse tumor differentiation, lymphatic metastasis, and advanced tumor stages. Paired analysis of tissues from the same patient further validated their inverse expression patterns during CRC progression. DNMT1-mediated DACH1 epigenetic silencing plays a critical role in CRC progression, suggesting that the DNMT1-DACH1 regulatory axis may serve as a potential biomarker and therapeutic target in CRC.
Keywords: DACH1; DNMT1; colorectal cancer; promoter methylation; tumor progression.
Copyright © 2025 Zhang and Liu.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Epigenetic regulation of DACH1, a novel Wnt signaling component in colorectal cancer.Epigenetics. 2013 Dec;8(12):1373-83. doi: 10.4161/epi.26781. Epub 2013 Oct 22. Epigenetics. 2013. PMID: 24149323 Free PMC article.
-
DNMT1 maintains the methylation of miR-152-3p to regulate TMSB10 expression, thereby affecting the biological characteristics of colorectal cancer cells.IUBMB Life. 2020 Nov;72(11):2432-2443. doi: 10.1002/iub.2366. Epub 2020 Sep 12. IUBMB Life. 2020. PMID: 32918845 Free PMC article.
-
Organoid modelling identifies that DACH1 functions as a tumour promoter in colorectal cancer by modulating BMP signalling.EBioMedicine. 2020 Jun;56:102800. doi: 10.1016/j.ebiom.2020.102800. Epub 2020 Jun 6. EBioMedicine. 2020. PMID: 32512510 Free PMC article.
-
Accumulation of aberrant DNA methylation during colorectal cancer development.World J Gastroenterol. 2014 Jan 28;20(4):978-87. doi: 10.3748/wjg.v20.i4.978. World J Gastroenterol. 2014. PMID: 24574770 Free PMC article. Review.
-
Aberrant protein glycosylation in the colon adenoma-cancer sequence: Colorectal cancer mechanisms and clinical implications.Biochim Biophys Acta Mol Basis Dis. 2025 Aug;1871(6):167853. doi: 10.1016/j.bbadis.2025.167853. Epub 2025 Apr 17. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40250777 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

