Qu-shi-hua-tan decoction's efficacy and safety for patients with angina following coronary revascularization: a randomized, double-blind, placebo-controlled trial study protocol
- PMID: 40371063
- PMCID: PMC12075204
- DOI: 10.3389/fcvm.2025.1512385
Qu-shi-hua-tan decoction's efficacy and safety for patients with angina following coronary revascularization: a randomized, double-blind, placebo-controlled trial study protocol
Abstract
Introduction: The Qu-shi-hua-tan decoction (QSHTD), formulated by academician Chen Keji, is an empirical decoction for coronary heart disease (CHD). We conducted a randomized controlled trial to assess the effectiveness and safety of QSHTD in managing angina after coronary revascularization (AACR) in CHD patients.
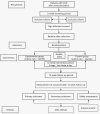
Methods and design: This double-blind randomized controlled trial will be conducted at Guangdong Provincial Hospital of Traditional Chinese Medicine. We will allocate 98 qualified participants to either the experimental or control group in a 1:1 ratio through random selection. The experimental group will be given standard care along with QSHTD, whereas the control group will receive standard care and a placebo. The study will span 26 weeks, consisting of a 2-week initial phase, a 12-week intervention phase, and a 12-week monitoring phase. The main outcome measure will be myocardial blood flow (MBF) assessed using adenosine stress real-time myocardial perfusion echocardiography (RTMPE). The secondary outcomes will be Canadian Cardiovascular Sociation Classification, Seattle Angina Questionnaire, Traditional Chinese Medicine (TCM) symptom evaluation; and major adverse cardiac events (MACE).
Discussion: This study seeks to deliver compelling proof of the superior methodological and reporting standards of QSHTD's effectiveness and safety within AACR treatment.
Clinical trial registration: Chinese Clinical Trial Registration Center [www.chictr.org.cn]. The trial was registered on November 26, 2020 [ChiCTR2000040270].
Keywords: Traditional Chinese Medicine; coronary heart disease; protocol; qu-shi-hua-tan decoction; randomized controlled trial.
© 2025 Xu, Wen, Li, Li, Zhang and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
-
- The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases in China 2022: an updated summary. Chin Circ J. (2023) 38(6):583–612.
-
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(3):e18–114. 10.1161/CIR.0000000000001038 - DOI - PubMed
LinkOut - more resources
Full Text Sources