Effects of naphthoquinone scaffold-derived compounds on head and neck squamous cell carcinoma based on network pharmacology and molecular docking
- PMID: 40371090
- PMCID: PMC12070129
- DOI: 10.62347/CMQJ5473
Effects of naphthoquinone scaffold-derived compounds on head and neck squamous cell carcinoma based on network pharmacology and molecular docking
Abstract
Objectives: This study aimed to analyze the effects of naphthoquinone scaffold-derived compounds on head and neck squamous cell carcinoma (HNSCC) using network pharmacology and molecular docking.
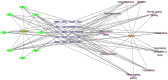
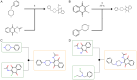
Methods: We screened candidate compounds from the ASINEX database and evaluated their drug likeness and toxicity. They identified 80 compounds with naphthalenone structures, focusing on 1,4-naphthoquinone and 1,2-naphthoquinone scaffolds. The possible targets of these compounds were predicted using databases like SwissTargetPrediction and Similarity Ensemble Approach Database (SEA).
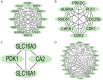
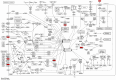
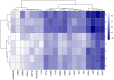
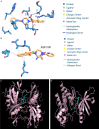
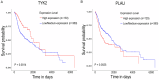
Results: The common targets between the compounds and HNSCC were identified, yielding 65 overlapping targets. A protein-protein interaction (PPI) network was constructed, and 20 hub genes were identified based on centrality metrics. Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that these compounds' protective effects against HNSCC are associated with cancer-related pathways, such as those in cancer and proteoglycans in cancer. Molecular docking was performed to evaluate the binding affinity between the compounds and hub genes. The results showed that the compounds had strong binding affinities with key targets like MET and TYK2, with binding energies < -5 kcal/mol.
Conclusions: The study suggests that naphthoquinone derivatives could serve as novel chemotherapy agents for HNSCC, warranting further research for clinical application.
Keywords: Naphthoquinone; head and neck squamous cell carcinoma; molecular docking; network pharmacology; virtual screening.
IJCEP Copyright © 2025.
Conflict of interest statement
None.
Figures


Similar articles
-
Analysis of action of 1,4-naphthoquinone scaffold-derived compounds against acute myeloid leukemia based on network pharmacology, molecular docking and molecular dynamics simulation.Sci Rep. 2024 Sep 9;14(1):21043. doi: 10.1038/s41598-024-70937-y. Sci Rep. 2024. PMID: 39251712 Free PMC article.
-
Molecular Mechanisms of Reversal of Multidrug Resistance in Breast Cancer by Inhibition of P-gp by Cytisine N-Isoflavones Derivatives Explored Through Network Pharmacology, Molecular Docking, and Molecular Dynamics.Int J Mol Sci. 2025 Apr 17;26(8):3813. doi: 10.3390/ijms26083813. Int J Mol Sci. 2025. PMID: 40332431 Free PMC article.
-
Identification of Molecular Targets and Potential Mechanisms of Yinchen Wuling San Against Head and Neck Squamous Cell Carcinoma by Network Pharmacology and Molecular Docking.Front Genet. 2022 Jul 6;13:914646. doi: 10.3389/fgene.2022.914646. eCollection 2022. Front Genet. 2022. PMID: 35873484 Free PMC article.
-
Network pharmacology and bioinformatics analysis on the underlying mechanisms of baicalein against oral squamous cell carcinoma.J Gene Med. 2023 Jun;25(6):e3490. doi: 10.1002/jgm.3490. Epub 2023 Mar 12. J Gene Med. 2023. PMID: 36843559
-
Integration of Network Pharmacology and Molecular Docking Technology Reveals the Mechanism of the Therapeutic Effect of Xixin Decoction on Alzheimer's Disease.Comb Chem High Throughput Screen. 2022;25(10):1785-1804. doi: 10.2174/1386207325666220523151119. Comb Chem High Throughput Screen. 2022. PMID: 35616676
References
-
- Lee YG, Kang EJ, Keam B, Choi JH, Kim JS, Park KU, Lee KE, Kwon JH, Lee KW, Kim MK, Ahn HK, Shin SH, Kim HR, Kim SB, Yun HJ. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective cohort study (KCSG HN13-01) BMC Cancer. 2020;20:813. - PMC - PubMed
-
- Crossman BE, Harmon RL, Kostecki KL, McDaniel NK, Iida M, Corday LW, Glitchev CE, Crow MT, Harris MA, Lin CY, Adams JM, Longhurst CA, Nickel KP, Ong IM, Alexandridis RA, Yu M, Yang DT, Hu R, Morris ZS, Hartig GK, Glazer TA, Ramisetty S, Kulkarni P, Salgia R, Kimple RJ, Bruce JY, Harari PM, Wheeler DL. From bench to bedside: a team’s approach to multidisciplinary strategies to combat therapeutic resistance in head and neck squamous cell carcinoma. J Clin Med. 2024;13:6036. - PMC - PubMed
-
- Zorzanelli BC, Ouverney G, Pauli FP, da Fonseca ACC, de Almeida ECP, de Carvalho DG, Possik PA, Rabelo VW, Abreu PA, Pontes B, Ferreira VF, Forezi L, da Silva FC, Robbs BK. Pro-apoptotic antitumoral effect of novel acridine-core naphthoquinone compounds against oral squamous cell carcinoma. Molecules. 2022;27:5148. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
