A Guide for Developing Demo-Genetic Models to Simulate Genetic Rescue
- PMID: 40371097
- PMCID: PMC12076008
- DOI: 10.1111/eva.70092
A Guide for Developing Demo-Genetic Models to Simulate Genetic Rescue
Abstract
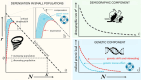
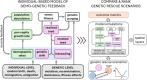
Genetic rescue is a conservation management strategy that reduces the negative effects of genetic drift and inbreeding in small and isolated populations. However, such populations might already be vulnerable to random fluctuations in growth rates (demographic stochasticity). Therefore, the success of genetic rescue depends not only on the genetic composition of the source and target populations but also on the emergent outcome of interacting demographic processes and other stochastic events. Developing predictive models that account for feedback between demographic and genetic processes ('demo-genetic feedback') is therefore necessary to guide the implementation of genetic rescue to minimize the risk of extinction of threatened populations. Here, we explain how the mutual reinforcement of genetic drift, inbreeding, and demographic stochasticity increases extinction risk in small populations. We then describe how these processes can be modelled by parameterizing underlying mechanisms, including deleterious mutations with partial dominance and demographic rates with variances that increase as abundance declines. We combine our suggestions of model parameterization with a comparison of the relevant capability and flexibility of five open-source programs designed for building genetically explicit, individual-based simulations. Using one of the programs, we provide a heuristic model to demonstrate that simulated genetic rescue can delay extinction of small virtual populations that would otherwise be exposed to greater extinction risk due to demo-genetic feedback. We then use a case study of threatened Australian marsupials to demonstrate that published genetic data can be used in one or all stages of model development and application, including parameterization, calibration, and validation. We highlight that genetic rescue can be simulated with either virtual or empirical sequence variation (or a hybrid approach) and suggest that model-based decision-making should be informed by ranking the sensitivity of predicted probability/time to extinction to variation in model parameters (e.g., translocation size, frequency, source populations) among different genetic-rescue scenarios.
Keywords: SLiM; conservation genetics; demography; density feedback; inbreeding depression; marsupials; software.
© 2025 The Author(s). Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The interaction of inbreeding depression and environmental stochasticity in the risk of extinction of small populations.EXS. 1994;68:131-46. doi: 10.1007/978-3-0348-8510-2_12. EXS. 1994. PMID: 8032131
-
Extinction vortex dynamics of top predators isolated by urbanization.Ecol Appl. 2019 Apr;29(3):e01868. doi: 10.1002/eap.1868. Epub 2019 Mar 20. Ecol Appl. 2019. PMID: 30892753
-
How density dependence, genetic erosion and the extinction vortex impact evolutionary rescue.Proc Biol Sci. 2023 Nov 29;290(2011):20231228. doi: 10.1098/rspb.2023.1228. Epub 2023 Nov 22. Proc Biol Sci. 2023. PMID: 37989246 Free PMC article.
-
Deleterious Variation in Natural Populations and Implications for Conservation Genetics.Annu Rev Anim Biosci. 2023 Feb 15;11:93-114. doi: 10.1146/annurev-animal-080522-093311. Epub 2022 Nov 4. Annu Rev Anim Biosci. 2023. PMID: 36332644 Free PMC article. Review.
-
Conservation and Genetics.Yale J Biol Med. 2018 Dec 21;91(4):491-501. eCollection 2018 Dec. Yale J Biol Med. 2018. PMID: 30588214 Free PMC article. Review.
References
-
- Benson, J. F. , Mahoney P. J., Sikich J. A., et al. 2016. “Interactions Between Demography, Genetics, and Landscape Connectivity Increase Extinction Probability for a Small Population of Large Carnivores in a Major Metropolitan Area.” Proceedings of the Royal Society B: Biological Sciences 283, no. 1837: 20160957. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

