Desulfovibrio vulgaris exacerbates sepsis by inducing inflammation and oxidative stress in multiple organs
- PMID: 40371102
- PMCID: PMC12075292
- DOI: 10.3389/fmicb.2025.1574998
Desulfovibrio vulgaris exacerbates sepsis by inducing inflammation and oxidative stress in multiple organs
Abstract
Introduction: Sepsis is a life-threatening condition that often leads to organ dysfunction and systemic inflammation, with gut microbiota dysbiosis playing a crucial role in its pathogenesis. The role of Desulfovibrio vulgaris (D. vulgaris), a potentially pathogenic bacterium, in sepsis remains unclear.
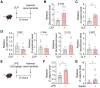
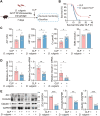
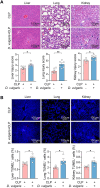
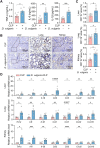
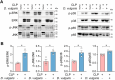
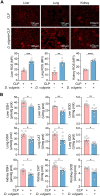
Methods: We first assessed the abundance of D. vulgaris in the feces of septic mice and patients using qPCR. Mice were then orally gavaged with D. vulgaris (2 × 108 CFU/mouse/day) for 7 consecutive days followed by cecal ligation and puncture (CLP) surgery. We monitored survival, assessed organ damage, and measured inflammation. Peritoneal macrophages were isolated to analyze the phosphorylation of key MAPK and NF-κB signaling pathways. Finally, oxidative stress levels in the liver, lungs, and kidneys were evaluated, measuring markers such as GSH, CAT, and SOD.
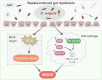
Results: The abundance of D. vulgaris was significantly increased in the feces of both septic mice and patients. Supplementation with D. vulgaris exacerbated sepsis in mice, resulting in lower survival rates, more severe organ damage, and heightened inflammation. Phosphorylation of MAPK and NF-κB pathways in peritoneal macrophages was significantly enhanced. Additionally, D. vulgaris amplified oxidative stress across multiple organs, as indicated by increased ROS levels and decreased antioxidant enzyme activity.
Conclusion: Our findings suggest that D. vulgaris exacerbates the progression of sepsis by enhancing inflammation, activating key immune signaling pathways, and increasing oxidative stress. These processes contribute to organ dysfunction and increased mortality, highlighting the potential pathogenic role of D. vulgaris in sepsis.
Keywords: Desulfovibrio vulgaris; inflammation; macrophage; oxidative stress; sepsis.
Copyright © 2025 Wu, Yu, Guo, Xiang, Zeng, Fu, Yang, Huang, Wang, Chen, Ge, Zhao and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Alpha-Chymotrypsin Protects Against Acute Lung, Kidney, and Liver Injuries and Increases Survival in CLP-Induced Sepsis in Rats Through Inhibition of TLR4/NF-κB Pathway.Drug Des Devel Ther. 2022 Sep 8;16:3023-3039. doi: 10.2147/DDDT.S370460. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36105322 Free PMC article.
-
Echinatin alleviates sepsis severity through modulation of the NF-κB and MEK/ERK signaling pathways.Biomed Pharmacother. 2024 Oct;179:117359. doi: 10.1016/j.biopha.2024.117359. Epub 2024 Sep 4. Biomed Pharmacother. 2024. PMID: 39236479
-
Klotho deficiency aggravates sepsis-related multiple organ dysfunction.Am J Physiol Renal Physiol. 2019 Mar 1;316(3):F438-F448. doi: 10.1152/ajprenal.00625.2017. Epub 2018 Dec 5. Am J Physiol Renal Physiol. 2019. PMID: 30516423
-
Overexpressing six-transmembrane protein of prostate 2 (STAMP2) alleviates sepsis-induced acute lung injury probably by hindering M1 macrophage polarization via the NF-κB pathway.Folia Histochem Cytobiol. 2023;61(1):34-46. doi: 10.5603/FHC.a2022.0032. Epub 2022 Dec 30. Folia Histochem Cytobiol. 2023. PMID: 36583372
-
Aquaporins in sepsis- an update.Front Immunol. 2024 Oct 31;15:1495206. doi: 10.3389/fimmu.2024.1495206. eCollection 2024. Front Immunol. 2024. PMID: 39544938 Free PMC article. Review.
References
-
- Bansal A., Mostafa M. M., Kooi C., Sasse S. K., Michi A. N., Shah S. V., et al. . (2022). Interplay between nuclear factor-kappaB, p38 MAPK, and glucocorticoid receptor signaling synergistically induces functional TLR2 in lung epithelial cells. J. Biol. Chem. 298:101747. doi: 10.1016/j.jbc.2022.101747, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

