Dalpiciclib plus aromatase inhibitor versus neoadjuvant chemotherapy for ER-positive, HER2-negative breast cancer
- PMID: 40371225
- PMCID: PMC12074949
- DOI: 10.3389/fonc.2025.1566146
Dalpiciclib plus aromatase inhibitor versus neoadjuvant chemotherapy for ER-positive, HER2-negative breast cancer
Abstract
Background: The combination of cyclin-dependent kinases 4 and 6 inhibitors (CDK4/6i) with endocrine therapy (ET) has emerged as an effective alternative to neoadjuvant chemotherapy (NCT) for patients with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2 (HER2) -negative breast cancer (BC). This single-center study retrospectively evaluated the efficacy and safety of dalpiciclib combined with an aromatase inhibitor (AI) compared to NCT.
Methods: The clinicopathological data and treatment details of patients with HR+ HER2 negative BC who underwent either neoadjuvant endocrine therapy (NET) or NCT were collected from the Fourth Hospital of Hebei Medical University. The primary endpoint of the study was the Residual Cancer Burden (RCB), while secondary endpoints included breast pathological complete response (bpCR), clinical response rates (ORR), proliferation markers, and safety profiles.
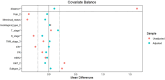
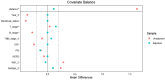
Results: Between May 2022 and June 2023, a total of 36 patients were treated with dalpiciclib plus AI, while 137 patients received NCT for the final analysis. Prior to propensity score matching (PSM), the rates of RCB 0 were 0% in the NET group and 7.3% in the NCT group (p=0.205). The rates of bpCR were 2.8% for the NET group and 13.1% for the NCT group (p=0.142). The ORR was comparable between the two groups (p=0.397), as were the rates of BCS (p=0.608). Both treatment groups demonstrated significant reductions in Ki-67 levels, although the extent of reduction was similar (p=0.174). Notably, a Ki-67 level of ≤ 10% post-treatment was more prevalent in the NET group (p<0.0001). Only two patients in the NET group achieved a Preoperative Endocrine Prognostic Index (PEPI) 0 score. The incidence of grade 3-4 toxicities was significantly higher in the NCT group compared to the NET group (p<0.05). Following PSM, patients treated with dalpiciclib plus AI exhibited comparable clinical responses and a safety advantage relative to those receiving NCT.
Conclusion: This study indicates that the combination of dalpiciclib and AI elicits comparable responses and demonstrates improved safety profiles when compared to NCT in patients with HR+ HER2 negative breast tumors. Furthermore, RCB and pCR may not serve as optimal endpoints for evaluating the efficacy of CDK4/6i-based NET.
Keywords: dalpiciclib; efficiency; hormone receptor positive/human epidermal growth factor receptor 2-negative breast cancer; neoadjuvant treatment; safety.
Copyright © 2025 Wang, Zhang, Gao, Li, Yang, Zhang, Wang, Li, Gao and Geng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Real-world comparison of palbociclib, abemaciclib, and dalpiciclib as first-line treatments for Chinese HR+/HER2-metastatic breast cancer patients: a multicenter study (YOUNGBC-28).Ther Adv Med Oncol. 2024 Dec 17;16:17588359241302018. doi: 10.1177/17588359241302018. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39697620 Free PMC article.
-
Dalpiciclib plus letrozole or anastrozole versus placebo plus letrozole or anastrozole as first-line treatment in patients with hormone receptor-positive, HER2-negative advanced breast cancer (DAWNA-2): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2023 Jun;24(6):646-657. doi: 10.1016/S1470-2045(23)00172-9. Epub 2023 May 11. Lancet Oncol. 2023. PMID: 37182538 Clinical Trial.
-
Comparative efficacy and safety of CDK4/6 inhibitors combined with endocrine therapies for HR+/HER2-breast cancer: Systematic review and network meta-analysis.Heliyon. 2024 May 21;10(11):e31583. doi: 10.1016/j.heliyon.2024.e31583. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38832268 Free PMC article.
-
Neoadjuvant Therapy of Cyclin-Dependent Kinase 4/6 Inhibitors Combined with Endocrine Therapy in HR+/HER2- Breast Cancer: A Systematic Review and Meta-Analysis.Oncol Res Treat. 2021;44(10):557-567. doi: 10.1159/000518573. Epub 2021 Aug 12. Oncol Res Treat. 2021. PMID: 34515204
-
Neoadjuvant chemotherapy combined with endocrine therapy for hormone receptor-positive breast cancer: A systematic review and meta-analysis.Medicine (Baltimore). 2023 Nov 17;102(46):e35928. doi: 10.1097/MD.0000000000035928. Medicine (Baltimore). 2023. PMID: 37986364 Free PMC article.
References
-
- Alba E, Calvo L, Albanell J, de la Haba JR, Lanza AA, Chacon JI, et al. . Chemotherapy (CT) and hormono therapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. (2012) 23:3069–74. doi: 10.1093/annonc/mds132 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous