Nasogastric Delivery of Fecal Microbiota Transplantation for the Treatment of Fulminant Clostridioides difficile Infection: A Case Report
- PMID: 40371237
- PMCID: PMC12075926
- DOI: 10.1002/jgh3.70177
Nasogastric Delivery of Fecal Microbiota Transplantation for the Treatment of Fulminant Clostridioides difficile Infection: A Case Report
Abstract
Introduction: Clostridioides difficile infection (CDI) is a significant cause of antibiotic-associated diarrhea with high morbidity and mortality, particularly in cases of fulminant disease. Fecal microbiota transplantation (FMT) has demonstrated efficacy in treating severe and refractory CDI, typically administered via colonoscopy. However, in cases complicated by toxic megacolon, alternative methods of FMT delivery may be necessary.
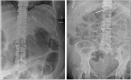
Case report: This case report describes a 46-year-old female with cirrhosis and fulminant CDI complicated by toxic megacolon. Due to the patient's hemodynamic instability and contraindications to endoscopic FMT delivery, a novel approach of nasogastric FMT administration was utilized. The patient received a combination of enema-delivered and nasogastric FMT alongside standard antibiotic therapy. This approach resulted in rapid clinical improvement, with resolution of toxic megacolon, normalization of inflammatory markers, and avoidance of colectomy.
Discussion: This report highlights the successful use of nasogastric FMT in a patient with fulminant CDI, offering a potential alternative delivery route when colonoscopic administration is contraindicated. To our knowledge, this is the first reported case of nasogastric FMT successfully resolving C. difficile-associated toxic megacolon.
Keywords: Clostridioides difficile infection (CDI); antibiotic‐associated diarrhea; fecal microbiota transplantation (FMT); nasogastric delivery; toxic megacolon.
© 2025 The Author(s). JGH Open: An open access journal of gastroenterology and hepatology published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Trubiano J. A., Cheng A. C., Korman T. M., et al., “Australasian Society of Infectious Diseases Updated Guidelines for the Management of Clostridium difficile Infection in Adults and Children in Australia and New Zealand,” Internal Medicine Journal 46, no. 4 (2016): 479–493, 10.1111/imj.13027. - DOI - PubMed
-
- Quraishi M. N., Widlak M., Bhala N., et al., “Systematic Review With Meta‐Analysis: The Efficacy of Faecal Microbiota Transplantation for the Treatment of Recurrent and Refractory Clostridium difficile Infection,” Alimentary Pharmacology & Therapeutics 46, no. 5 (2017): 479–493, 10.1111/apt.14201. - DOI - PubMed
LinkOut - more resources
Full Text Sources