Efficient synthesis of O-glycosylated amino acids
- PMID: 40371313
- PMCID: PMC12070420
- DOI: 10.1039/d5cb00076a
Efficient synthesis of O-glycosylated amino acids
Abstract
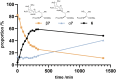
Protein glycosylation is one of the most abundant and complex post-translational modifications, necessitating many different approaches to fully understand the biological effects. Investigation using synthetic glycopeptides is limited by the high cost of building blocks; typically >100x more than other modified amino acids e.g. phosphorylation. We report a simple, low cost route to O-glycosylated amino acids suitable for Fmoc-SPPS in two or three steps starting from peracetylated sugars. One set of reagents can furnish either the α- or β-anomer through adjusting the equivalents and reaction time. Depending on the derivative, the cost of our route is 25-60× less than commercial alternatives and offers scope for producing modified analogues. Overall, this is a convenient and user-friendly approach to access O-glycosylated amino acids, urgently required for continued investigation of the manifold roles of glycosylation in biology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources

