Post-marketing safety of anakinra and canakinumab: a real-world pharmacovigilance study based on FDA adverse event reporting system
- PMID: 40371323
- PMCID: PMC12075943
- DOI: 10.3389/fphar.2025.1483669
Post-marketing safety of anakinra and canakinumab: a real-world pharmacovigilance study based on FDA adverse event reporting system
Abstract
Background: Anakinra and canakinumab are two FDA-approved IL-1 blockers indicated for the treatment of multiple autoinflammatory diseases, yet their safety has not been comprehensively analyzed. We aimed to assess the safety signals associated with anakinra and canakinumab by conducting a pharmacovigilance analysis using the FDA Adverse Event Reporting System (FAERS) database.
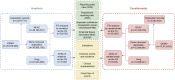
Methods: Adverse reaction data spanning from the first quarter of 2004 to the fourth quarter of 2023 was downloaded from the FAERS database. A disproportionality analysis utilizing various methods, including the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and Empirical Bayes Geometric Mean (EBGM), was conducted.
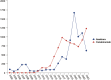
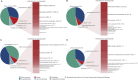
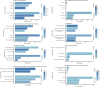
Results: Anakinra and canakinumab were identified as the primary suspect drugs for adverse events (AEs) in 7,544 and 8,044 reports, respectively. The most commonly reported SOCs for both drugs were general disorders and administration site conditions. Subgroup analyses indicated that the most commonly reported SOC signals among health professionals and non-health professionals remained consistent across both medications. At the preferred term (PT) level, consistent with the drug labeling, the common AEs for anakinra and canakinumab included injection site reactions (ISRs) and infections. Further analysis revealed a higher frequency of ISRs with anakinra, including injection site pain and erythema. In contrast, canakinumab was associated with more gastrointestinal disorders (abdominal pain, mouth ulceration, and inflammatory bowel disease) and respiratory disorders (cough, oropharyngeal pain, and rhinorrhea); these conditions predominantly occurred among minors. Notably, no significant safety signals related to tuberculosis infection or reactivation were observed, and the frequency of AEs related to hepatic injury and malignancy was low.
Conclusion: This study confirms the favorable safety profiles of anakinra and canakinumab, offering critical insights into rational drug usage and safety regulations.
Keywords: FAERS database; IL-1; adverse events; anakinra; autoinflammatory diseases; canakinumab; pharmacovigilance; real-world data analysis.
Copyright © 2025 Liu, Yan, Li, Yan and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Post-market safety profile of cefiderocol: a real-world pharmacovigilance exploratory analysis based on U.S. FDA adverse event reporting system (FAERS).BMC Pharmacol Toxicol. 2025 Mar 11;26(1):58. doi: 10.1186/s40360-025-00894-3. BMC Pharmacol Toxicol. 2025. PMID: 40069825 Free PMC article.
-
Suspected adverse drug reactions of rivaroxaban reported in the United States food and drug administration adverse event reporting system database: a pharmacovigilance study.Front Pharmacol. 2024 Sep 6;15:1399172. doi: 10.3389/fphar.2024.1399172. eCollection 2024. Front Pharmacol. 2024. PMID: 39309013 Free PMC article.
-
Safety assessment of deutetrabenazine: real-world adverse event analysis from the FAERS database.Front Pharmacol. 2024 Dec 23;15:1498215. doi: 10.3389/fphar.2024.1498215. eCollection 2024. Front Pharmacol. 2024. PMID: 39764469 Free PMC article.
-
Romosozumab adverse event profile: a pharmacovigilance analysis based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023.Aging Clin Exp Res. 2025 Jan 14;37(1):23. doi: 10.1007/s40520-024-02921-5. Aging Clin Exp Res. 2025. PMID: 39808360 Free PMC article.
-
A Systematic Review of the Safety of Blocking the IL-1 System in Human Pregnancy.J Clin Med. 2021 Dec 31;11(1):225. doi: 10.3390/jcm11010225. J Clin Med. 2021. PMID: 35011965 Free PMC article. Review.
References
-
- Atas N., Eroglu G. A., Sodan H. N., Ozturk B. O., Babaoglu H., Satis H., et al. (2021). Long-term safety and efficacy of anakinra and canakinumab in patients with familial Mediterranean fever: a single-centre real-life study with 101 patients. Clin. Exp. Rheumatol. 132 (5), 30–36. 10.55563/clinexprheumatol/815tdt - DOI - PubMed
LinkOut - more resources
Full Text Sources

