Huangkui capsules regulate tryptophan metabolism to improve diabetic nephropathy through the Keap1/Nrf2/HO-1 pathway
- PMID: 40371325
- PMCID: PMC12075421
- DOI: 10.3389/fphar.2025.1535352
Huangkui capsules regulate tryptophan metabolism to improve diabetic nephropathy through the Keap1/Nrf2/HO-1 pathway
Abstract
Background: Diabetic nephropathy (DN) is a serious complication of diabetes and one of the leading causes of end-stage renal disease. Huangkui capsule (HKC), a traditional Chinese patent medicine, is widely used in clinical practice for the treatment of chronic glomerulonephritis. However, the therapeutic effects and underlying mechanisms of HKC in DN remain poorly understood.
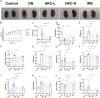
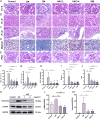
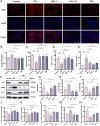
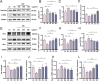
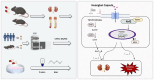
Methods: DN was induced in db/db mice, which were randomly divided into the DN, HKC-L, HKC-H and IRB groups, and db/m mice served as the Control group. Biochemical indices of blood and urine samples from the mice were measured, and HE staining, Masson staining and PAS staining were used to verify the anti-DN effect of HKC. The levels of ROS and the expression of Nrf2 pathway-related proteins and mRNAs were detected. Metabonomic analysis was used to investigate the role of tryptophan metabolism in the regulation of DN by HKC. HK-2 cells were used to establish a model of high-glucose (HG) injury in vitro, and HKC treatment was given for supplementary verification. Sarpogrelate hydrochloride (SH) combined with HKC, a 5-HT2AR inhibitor, was used to verify the effect of the 5-HT pathway in an in vitro model.
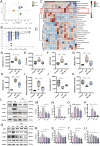
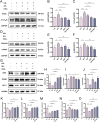
Results: Treatment with HKC significantly inhibited the increase in blood glucose and Urinary albumin/creatinine ratio (UACR), improved kidney injury signs in mice, reduced the level of ROS and improved oxidative stress injury through the Keap1/ Nrf2/HO-1 pathway. Metabonomic analysis revealed that tryptophan metabolism is involved in the process by which HKC improves DN, and HKC can regulate the 5-HT pathway to improve the renal injury by oxidative stress regulation. HKC treatment also significantly improved the renal and oxidative stress injuries in HG HK-2 cell model through the Nrf2 pathway in vitro. SH administration revealed that inhibiting 5-HT2AR could significantly inhibit the synthesis of 5-HT and improve the renal injury induced by HG.
Conclusion: Our study demonstrate that HKC can inhibit kidney injury and oxidative stress injury in db/db mice and HK-2 cells by regulating tryptophan metabolism and the Keap1/Nrf2/HO-1 pathway, which provides new insight for the clinical use of HKC for treatment of DN.
Keywords: Keap1/ Nrf2/HO-1 pathway; diabetic nephropathy; huangkui capsules; oxidative stress; tryptophan metabolism.
Copyright © 2025 Su, Zhang, Wang, Hu, Zhou, Zhu, Liu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway.J Ethnopharmacol. 2021 Dec 5;281:113548. doi: 10.1016/j.jep.2020.113548. Epub 2020 Nov 3. J Ethnopharmacol. 2021. PMID: 33152427
-
Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid.J Ethnopharmacol. 2015 Sep 15;173:256-65. doi: 10.1016/j.jep.2015.07.036. Epub 2015 Jul 28. J Ethnopharmacol. 2015. PMID: 26226437
-
Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats.J Ethnopharmacol. 2016 Aug 2;189:238-49. doi: 10.1016/j.jep.2016.05.033. Epub 2016 May 17. J Ethnopharmacol. 2016. PMID: 27224243
-
Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management.Acta Pharm Sin B. 2021 Sep;11(9):2749-2767. doi: 10.1016/j.apsb.2020.12.020. Epub 2021 Feb 2. Acta Pharm Sin B. 2021. PMID: 34589395 Free PMC article. Review.
-
Efficacy and safety of Huangkui capsule for diabetic nephropathy: A systematic review and meta-analysis.Medicine (Baltimore). 2024 May 31;103(22):e38417. doi: 10.1097/MD.0000000000038417. Medicine (Baltimore). 2024. PMID: 39259064 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

