Nanotechnology-driven strategies in postoperative cancer treatment: innovations in drug delivery systems
- PMID: 40371327
- PMCID: PMC12075547
- DOI: 10.3389/fphar.2025.1586948
Nanotechnology-driven strategies in postoperative cancer treatment: innovations in drug delivery systems
Abstract
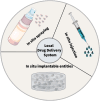
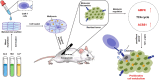
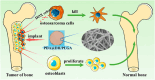
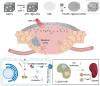
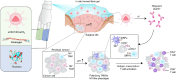
Cancer remains a global health challenge, and this challenge comes with a significant burden. Current treatment modalities, such as surgery, chemotherapy, and radiotherapy, have their limitations. The emergence of nanomedicines presents a new frontier in postoperative cancer treatment, offering potential to inhibit tumor recurrence and manage postoperative complications. This review deeply explores the application and potential of nanomedicines in the treatment of cancer after surgery. In particular, it focuses on local drug delivery systems (LDDS), which consist of in situ injection, implantation, and spraying. LDDS can provide targeted drug delivery and controlled release, which enhancing therapeutic efficacy. At the same time, it minimizes damage to healthy tissues and reduces systemic side effects. The nanostructures of these systems are unique. They facilitate the sustained release of drugs, prolong the effects of treatment, and decrease the frequency of dosing. This is especially beneficial in the postoperative period. Despite their potential, nanomedicines have limitations. These include high production costs, concerns regarding long-term toxicity, and complex regulatory approval processes. This paper aims to analyze several aspects. These include the advantages of nanomedicines, their drug delivery systems, how they combine with multiple treatment methods, and the associated challenges. Future research should focus on certain issues. These issues are stability, tumor specificity, and clinical translation. By addressing these, the delivery methods can be optimized and their therapeutic efficacy enhanced. With the advancements in materials science and biomedical engineering, the future design of LDDS is set to become more intelligent and personalized. It will cater to the diverse needs of clinical treatment and offer hope for better outcomes in cancer patients after surgery.
Keywords: anti-cancer; combination therapies; local drug delivery system; nanomedicines; postoperative treatment.
Copyright © 2025 Zhou, Feng, Zhao, Guo and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources

