Pulmonary artery-targeted low-dose metformin-loaded nanocapsules safely improve pulmonary arterial hypertension in rats
- PMID: 40371328
- PMCID: PMC12075939
- DOI: 10.3389/fphar.2025.1577570
Pulmonary artery-targeted low-dose metformin-loaded nanocapsules safely improve pulmonary arterial hypertension in rats
Abstract
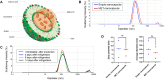
Introduction: Pulmonary arterial hypertension (PAH) remains a challenge to tackle despite various available medications. Metformin, although promising, has major adverse effects; the use of an appropriate drug delivery method may improve its efficacy and safety. The aim of this study was to develop a novel treatment for PAH using metformin. We developed a novel approach of using low-dose metformin encapsulated in pulmonary artery-targeted nanocapsules to alleviate PAH while avoiding adverse effects.
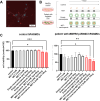
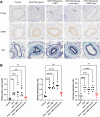
Methods: Metformin-loaded lung-targeted nanocapsules (MET nanocapsules) were created using a specific lipid composition, including cationic lipids. Their uptake and effects on cell viability were assessed in human pulmonary arterial smooth muscle cells (hPASMCs) from healthy individuals and patients with PAH. Their therapeutic effects were assessed in a PAH rat model. The safety of MET nanocapsules was confirmed using rat serum biochemical tests.
Results: We successfully prepared MET nanocapsules and demonstrated their effectiveness in inhibiting PASMC proliferation. In PAH model rats, MET nanocapsule treatment led to improved hemodynamics, right ventricular hypertrophy, and pulmonary arterial medial thickening. The nanocapsules effectively accumulated in the lungs of PAH model rats.
Conclusion: Intravenous administration of MET nanocapsules is a safe and innovative therapeutic approach for PAH. This method could improve PAH treatment outcomes while minimizing adverse effects, with potential applications in other types of pulmonary hypertension.
Keywords: drug delivery system; liposome; metformin; nanocapsule; pulmonary arterial hypertension.
Copyright © 2025 Chida-Nagai, Masaki, Sato, Kato, Takakuwa, Matsuno, Manabe and Takeda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension.Br J Pharmacol. 2009 Nov;158(5):1285-94. doi: 10.1111/j.1476-5381.2009.00445.x. Epub 2009 Oct 8. Br J Pharmacol. 2009. PMID: 19814724 Free PMC article.
-
Oral delivery of ambrisentan-loaded lipid-core nanocapsules as a novel approach for the treatment of pulmonary arterial hypertension.Int J Pharm. 2021 Dec 15;610:121181. doi: 10.1016/j.ijpharm.2021.121181. Epub 2021 Oct 12. Int J Pharm. 2021. PMID: 34653563
-
Plasminogen activator Inhibitor-2 inhibits pulmonary arterial smooth muscle cell proliferation in pulmonary arterial hypertension via PI3K/Akt and ERK signaling.Exp Cell Res. 2021 Jan 1;398(1):112392. doi: 10.1016/j.yexcr.2020.112392. Epub 2020 Nov 21. Exp Cell Res. 2021. PMID: 33227315
-
Contribution of transient receptor potential canonical channels in human and experimental pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2023 Aug 1;325(2):L246-L261. doi: 10.1152/ajplung.00011.2023. Epub 2023 Jun 27. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 37366608
-
Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension.Respir Res. 2023 Nov 1;24(1):263. doi: 10.1186/s12931-023-02559-3. Respir Res. 2023. PMID: 37915044 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

