Regulating mitochondrial oxidative phosphorylation and MAPK signaling: wedelolactone as a novel therapeutic for radiation-induced thrombocytopenia
- PMID: 40371333
- PMCID: PMC12075257
- DOI: 10.3389/fphar.2025.1508215
Regulating mitochondrial oxidative phosphorylation and MAPK signaling: wedelolactone as a novel therapeutic for radiation-induced thrombocytopenia
Abstract
Introduction: Radiation-induced thrombocytopenia (RIT) is a serious complication of cancer radiotherapy, for which therapeutic options are limited. This study investigates wedelolactone (WED), a metabolite of a botanical drug, as a potential treatment for RIT.
Methods: In vitro experiments were conducted using Meg-01 and K562 cell lines to evaluate the effects of WED on megakaryocyte differentiation and maturation. Flow cytometry and phalloidin staining were employed to assess the expression of megakaryocyte-specific markers CD41 and CD61, as well as nuclear polyploidization. A mouse model of RIT was established to assess the efficacy of WED in restoring platelet counts and regulating hematopoiesis. RNA sequencing and western blot analyses were performed to explore the underlying molecular mechanisms.
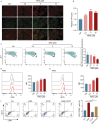
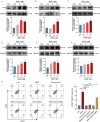
Results: In vitro experiments revealed that WED enhanced megakaryocyte differentiation in a dose-dependent manner, increasing the expression of lineage-specific markers CD41 and CD61, and promoting polyploidization and cytoskeletal reorganization. In vivo, WED significantly restored platelet counts in the mouse model of RIT and promoted the production of hematopoietic stem cells (HSCs), megakaryocytes, and reticulated platelets. RNA sequencing and western blot revealed that WED-induced megakaryocyte differentiation involves the regulation of mitochondrial oxidative phosphorylation mediated by the AMPK signaling pathway and activation of the MAPK signaling pathway. Inhibition of mitochondrial oxidative phosphorylation or MAPK signaling suppressed WED-induced megakaryocyte differentiation, highlighting the central role of these pathways.
Discussion: These findings indicate that WED could be a promising therapeutic candidate for RIT, acting through the modulation of oxidative phosphorylation and MAPK signaling pathways to enhance thrombopoiesis.
Keywords: megakaryocyte differentiation; oxidative phosphorylation; thrombocytopenia; thrombopoiesis; wedelolactone.
Copyright © 2025 Li, Li, Wu, Mei, Qi, Liu, Qiao, Shen, Luo, Zeng, Huang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Multimodal discovery of bavachinin A: A natural FLT3 agonist for treating thrombocytopenia.Phytomedicine. 2025 May;140:156597. doi: 10.1016/j.phymed.2025.156597. Epub 2025 Mar 1. Phytomedicine. 2025. PMID: 40058315
-
Xanthotoxin, a novel inducer of platelet formation, promotes thrombocytopoiesis via IL-1R1 and MEK/ERK signaling.Biomed Pharmacother. 2023 Jul;163:114811. doi: 10.1016/j.biopha.2023.114811. Epub 2023 May 8. Biomed Pharmacother. 2023. PMID: 37156117
-
Discovery of a novel megakaryopoiesis enhancer, ingenol, promoting thrombopoiesis through PI3K-Akt signaling independent of thrombopoietin.Pharmacol Res. 2022 Mar;177:106096. doi: 10.1016/j.phrs.2022.106096. Epub 2022 Jan 22. Pharmacol Res. 2022. PMID: 35077844
-
Megakaryocyte modification of platelets in thrombocytopenia.Curr Opin Hematol. 2018 Sep;25(5):410-415. doi: 10.1097/MOH.0000000000000451. Curr Opin Hematol. 2018. PMID: 29985173 Review.
-
The Application of Ethnomedicine in Modulating Megakaryocyte Differentiation and Platelet Counts.Int J Mol Sci. 2023 Feb 5;24(4):3168. doi: 10.3390/ijms24043168. Int J Mol Sci. 2023. PMID: 36834579 Free PMC article. Review.
References
-
- Chase T. H., Lyons B. L., Bronson R. T., Foreman O., Donahue L. R., Burzenski L. M., et al. (2010). The mouse mutation “thrombocytopenia and cardiomyopathy” (trac) disrupts Abcg5: a spontaneous single gene model for human hereditary phytosterolemia/sitosterolemia. Blood 115 (6), 1267–1276. 10.1182/blood-2009-05-219808 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

