Inhibition of NMDA receptors and other ion channel types by membrane-associated drugs
- PMID: 40371334
- PMCID: PMC12075551
- DOI: 10.3389/fphar.2025.1561956
Inhibition of NMDA receptors and other ion channel types by membrane-associated drugs
Abstract
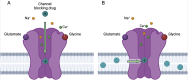
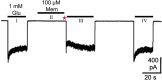
N-methyl-D-aspartate receptors (NMDARs) are ligand-gated ion channels present at most excitatory synapses in the brain that play essential roles in cognitive functions including learning and memory consolidation. However, NMDAR dysregulation is implicated in many nervous system disorders. Diseases that involve pathological hyperactivity of NMDARs can be treated clinically through inhibition by channel blocking drugs. NMDAR channel block can occur via two known mechanisms. First, in traditional block, charged drug molecules can enter the channel directly from the extracellular solution after NMDAR activation and channel opening. Second, uncharged molecules of channel blocking drug can enter the hydrophobic plasma membrane, and upon NMDAR activation the membrane-associated drug can transit into the channel through a fenestration within the NMDAR. This membrane-associated mechanism of action is called membrane to channel inhibition (MCI) and is not well understood despite the clinical importance of NMDAR channel blocking drugs. Intriguingly, a hydrophobic route of access for drugs is not unique to NMDARs. Our review will address inhibition of NMDARs and other ion channels by membrane-associated drugs and consider how the path of access may affect a drug's therapeutic potential.
Keywords: MCI; NMDAR; channel block; hydrophobic; ketamine; memantine; membrane.
Copyright © 2025 Neureiter, Erickson-Oberg, Nigam and Johnson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Inhibition of NMDA receptors through a membrane-to-channel path.Nat Commun. 2022 Jul 15;13(1):4114. doi: 10.1038/s41467-022-31817-z. Nat Commun. 2022. PMID: 35840593 Free PMC article.
-
Memantine and Ketamine Differentially Alter NMDA Receptor Desensitization.J Neurosci. 2017 Oct 4;37(40):9686-9704. doi: 10.1523/JNEUROSCI.1173-17.2017. Epub 2017 Sep 6. J Neurosci. 2017. PMID: 28877967 Free PMC article.
-
The chemical biology of clinically tolerated NMDA receptor antagonists.J Neurochem. 2006 Jun;97(6):1611-26. doi: 10.1111/j.1471-4159.2006.03991.x. J Neurochem. 2006. PMID: 16805772 Review.
-
Effects of Mg2+ on recovery of NMDA receptors from inhibition by memantine and ketamine reveal properties of a second site.Neuropharmacology. 2018 Jul 15;137:344-358. doi: 10.1016/j.neuropharm.2018.05.017. Epub 2018 May 12. Neuropharmacology. 2018. PMID: 29793153 Free PMC article.
-
N-[2-(N-(2-mercaptoethyl)) amino ethyl]-N-(2-mercaptoethyl)-3,5-dimethylacetamide amantadine-technetium.2012 Jun 12 [updated 2012 Jul 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jun 12 [updated 2012 Jul 17]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22812023 Free Books & Documents. Review.
References
-
- Alaia M. J., Hurley E. T., Vasavada K., Markus D. H., Britton B., Gonzalez-Lomas G., et al. (2022). Buccally absorbed cannabidiol shows significantly superior pain control and improved satisfaction immediately after arthroscopic rotator cuff repair: a placebo-controlled, double-blinded, randomized trial. Am. J. Sports Med. 50 (11), 3056–3063. 10.1177/03635465221109573 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

