Antidepressant-like activity of Bezafibrate in mice models of depression: a behavioral and neurobiological characterization
- PMID: 40371339
- PMCID: PMC12075530
- DOI: 10.3389/fphar.2025.1595341
Antidepressant-like activity of Bezafibrate in mice models of depression: a behavioral and neurobiological characterization
Abstract
Background: Depression represents a major global public health challenge, inflicting profound suffering on patients while imposing substantial socioeconomic burdens on families and healthcare systems. Although monoamine-based antidepressants remain first-line pharmacotherapy, accumulating clinical evidence reveals several limitations of these medications, including delayed pharmacodynamics and low remission rates. Therefore, it is necessary to search for new drugs and develop effective strategies for depression treatment. Bezafibrate (BEZ), which can activate proliferator-activated receptor a (PPARα), exhibit various biological functions, such as improving mitochondrial function, reducing neuroinflammation, and improving cognitive function. This study is to explore whether BEZ has antidepressant-like effects and its potential mechanisms.
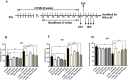
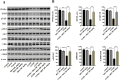
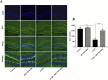
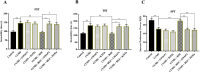
Methods: The antidepressant effects and potential mechanisms of BEZ were assessed by using forced swim test, tail suspension test, sucrose preference test, Western blot, gene interference, and immunofluorescence in the chronic unpredictable mild stress (CUMS) models of depression.
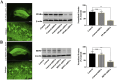
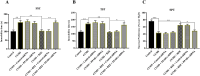
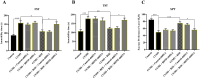
Results: Results showed that BEZ treatment significantly reversed depressive behavior in CUMS mice. The administration of BEZ obviously promoted the expression of PPAR, enhanced the BDNF signaling pathway, promoted hippocampal neurogenesis in CUMS mice. In addition, the pharmacologcial inhibitors GW6471 and K252a were obviously prevented the antidepressant effect of BEZ. Furthermore, gene knockdown of hippocampal PPARα or BDNF by using AAV-PPARα-shRNA-EGFP and AAV-BDNF-shRNA-EGFP, can remarkably inhibit the antidepressant effect of BEZ.
Conclusion: Collectively, the behavioral and neurobiological results demonstrate that BEZ exhibits antidepressant-like activity through PPARα/BDNF signaling pathway and may use as a potential antidepressant.
Keywords: BNDF; Bezafibrate; CUMS; PPARα; depression.
Copyright © 2025 Xu, Zhou, Zhou, Wang, Wang, Jiang and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Administration of chiglitazar reverses chronic stress-induced depressive-like symptoms in mice via activation of hippocampal PPARα and BDNF.Front Pharmacol. 2025 Apr 28;16:1587399. doi: 10.3389/fphar.2025.1587399. eCollection 2025. Front Pharmacol. 2025. PMID: 40356991 Free PMC article.
-
Antidepressant-like activity of oroxylin A in mice models of depression: A behavioral and neurobiological characterization.Front Pharmacol. 2022 Jul 26;13:921553. doi: 10.3389/fphar.2022.921553. eCollection 2022. Front Pharmacol. 2022. PMID: 35959431 Free PMC article.
-
Antidepressant-like activity of L-701324 in mice: A behavioral and neurobiological characterization.Behav Brain Res. 2021 Feb 5;399:113038. doi: 10.1016/j.bbr.2020.113038. Epub 2020 Dec 1. Behav Brain Res. 2021. PMID: 33276033
-
The antidepressant-like effects of the water extract of Panax ginseng and Polygala tenuifolia are mediated via the BDNF-TrkB signaling pathway and neurogenesis in the hippocampus.J Ethnopharmacol. 2021 Mar 1;267:113625. doi: 10.1016/j.jep.2020.113625. Epub 2020 Nov 25. J Ethnopharmacol. 2021. PMID: 33248184
-
The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside.Brain Res. 2024 Nov 15;1843:149123. doi: 10.1016/j.brainres.2024.149123. Epub 2024 Jul 16. Brain Res. 2024. PMID: 39025397 Review.
References
-
- Alzarea S., Rahman S. (2025). The alpha-7 nicotinic Receptor positive allosteric modulator PNU120596 attenuates lipopolysaccharide-induced depressive-like behaviors and cognitive impairment by regulating the PPAR-α signaling pathway in mice. CNS Neurol. Disord. Drug Targets 24, 234–244. 10.2174/0118715273311527240916050749 - DOI - PubMed
LinkOut - more resources
Full Text Sources

