Acrylamide and bisphenol A: two plastic additives increase platelet activation, via oxidative stress
- PMID: 40371341
- PMCID: PMC12075958
- DOI: 10.3389/fphar.2025.1526374
Acrylamide and bisphenol A: two plastic additives increase platelet activation, via oxidative stress
Abstract
Background: Since the mid-20th century, the widespread use of plastics has led to the buildup of harmful byproducts in the environment-most notably acrylamide (AA) and bisphenol A (BPA). These chemicals are now commonly detected in human tissues, raising concerns about their potential health effects. While their presence as environmental pollutants is well known, their specific impact on platelet function and the associated cardiovascular risks remains poorly understood.
Methods: To explore how AA and BPA affect platelet physiology, we performed in vitro assays to assess platelet activation and aggregation following exposure to these compounds. We also used bioinformatic tools to identify potential protein targets in human platelets and carried out molecular docking simulations to investigate how AA and BPA interact with key enzymes involved in platelet regulation.
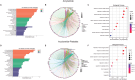
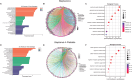
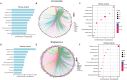
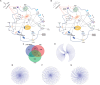
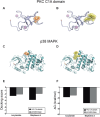
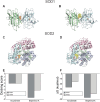
Results: Both AA and BPA exposure led to a significant increase in platelet activation and aggregation, suggesting an elevated risk of thrombosis. Proteomic analysis identified around 1,230 potential protein targets, with 191 affected by AA and 429 by BPA. These proteins are primarily involved in oxidative stress, apoptosis, and signaling pathways regulated by protein kinase C (PKC), p38α-MAPK, and superoxide dismutase (SOD). Molecular modeling further revealed that AA and BPA form stable complexes with several of these enzymes, indicating direct interference with platelet function.
Discussion and conclusion: Our study shows that AA and BPA can enhance platelet reactivity and aggregation, which are key factors in the development of cardiovascular disease (CVD). By identifying specific molecular pathways and targets affected by these pollutants, we provide new insights into their potential role in promoting thrombotic conditions. These findings highlight the urgent need for greater public health awareness and stronger regulatory efforts to reduce human exposure to AA and BPA.
Keywords: acrylamide; bisphenol; cardiovascular diseases; microplastics; platelets; platelets and cardiovascular diseases.
Copyright © 2025 Burgos, Méndez, Quintana, Gonkowski, Trostchansky and Alarcón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
-
Exploring the mechanisms underlying effects of bisphenol a on cardiovascular disease by network toxicology and molecular docking.Heliyon. 2024 May 17;10(10):e31473. doi: 10.1016/j.heliyon.2024.e31473. eCollection 2024 May 30. Heliyon. 2024. PMID: 38813174 Free PMC article.
-
Network toxicology and molecular docking techniques to explore the mechanism of bisphenol A on obesity.Int J Environ Health Res. 2025 Apr 19:1-13. doi: 10.1080/09603123.2025.2494735. Online ahead of print. Int J Environ Health Res. 2025. PMID: 40252053
-
Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway.Life Sci. 2021 Nov 1;284:119878. doi: 10.1016/j.lfs.2021.119878. Epub 2021 Aug 10. Life Sci. 2021. PMID: 34384828
-
Bacterial bioremediation as a sustainable strategy for the mitigation of Bisphenol-A.Environ Geochem Health. 2024 Aug 21;46(10):386. doi: 10.1007/s10653-024-02154-5. Environ Geochem Health. 2024. PMID: 39167247 Review.
References
LinkOut - more resources
Full Text Sources

