Macrophage-driven exosomes regulate the progression of cardiovascular disease
- PMID: 40371346
- PMCID: PMC12075947
- DOI: 10.3389/fphar.2025.1563800
Macrophage-driven exosomes regulate the progression of cardiovascular disease
Abstract
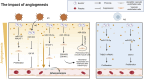
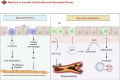
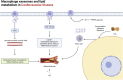
Exosomes, as vital mediators of intercellular communication, play a critical role in the progression of cardiovascular disease (CVD). Recently, macrophage-derived exosomes (Mφ-Exos) have garnered increasing attention because of their significant potential in early diagnosis, pathological processes, and therapeutic applications for CVD. Exosomes contain diverse nucleic acids (e.g., miRNAs, mRNAs, and long noncoding RNAs (lncRNAs)) and proteins, which serve as specific biomarkers that regulate various stages of CVD. For example, miRNAs encapsulated within exosomes (e.g., miR-21, miR-133a, and miR-155) are closely associated with atherosclerosis, myocardial infarction, coronary artery disease, and stroke, and changes in their abundance can serve as diagnostic and prognostic indicators. Additionally, the composition of Mφ-Exos, including miRNAs, lipids, and proteins, plays a significant role in the initiation, progression, and inflammation of CVD. Research on Mφ-Exos provides new directions for early diagnosis, mechanistic exploration, and novel therapeutic targets in CVD. However, challenges remain regarding exosome isolation and identification technologies. Future studies need to further explore the biological properties of exosomes and develop more efficient, economical, and straightforward isolation methods. This review summarizes the multifaceted regulatory roles of Mφ-Exos in CVD, encompassing key processes such as inflammation, angiogenesis, metabolism, and cell death. Research has shown that M1-Exos promote the progression and exacerbation of CVD through pro-inflammatory and pro-fibrotic mechanisms, while M2-Exos demonstrate significant therapeutic potential via anti-inflammatory, pro-angiogenic, and metabolic reprogramming pathways. These findings not only reveal the complex mechanisms of Mφ-Exos in CVD but also provide new perspectives and potential targets for early diagnosis and precision treatment of the disease.
Keywords: cardiovascular disease; exosomes; extracellular vesicles; inflamation; macrophage polarization.
Copyright © 2025 Qi, Luo, Li, Yan and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF.Stem Cell Res Ther. 2022 Apr 8;13(1):149. doi: 10.1186/s13287-022-02823-1. Stem Cell Res Ther. 2022. PMID: 35395782 Free PMC article.
-
Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury.Exp Neurol. 2023 Apr;362:114295. doi: 10.1016/j.expneurol.2022.114295. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493861
-
Endothelial progenitor cell-derived exosomes promote anti-inflammatory macrophages via SOCS3/JAK2/STAT3 axis and improve the outcome of spinal cord injury.J Neuroinflammation. 2023 Jun 30;20(1):156. doi: 10.1186/s12974-023-02833-7. J Neuroinflammation. 2023. PMID: 37391774 Free PMC article.
-
Unraveling the Intricate Roles of Exosomes in Cardiovascular Diseases: A Comprehensive Review of Physiological Significance and Pathological Implications.Int J Mol Sci. 2023 Oct 27;24(21):15677. doi: 10.3390/ijms242115677. Int J Mol Sci. 2023. PMID: 37958661 Free PMC article. Review.
-
Therapeutic Applications of Stem Cell-Derived Exosomes.Int J Mol Sci. 2024 Mar 21;25(6):3562. doi: 10.3390/ijms25063562. Int J Mol Sci. 2024. PMID: 38542535 Free PMC article. Review.
References
-
- Albrecht M., Hummitzsch L., Rusch R., Eimer C., Rusch M., Heß K., et al. (2023a). Large extracellular vesicles derived from human regulatory macrophages (L-EV(Mreg)) attenuate CD3/CD28-induced T-cell activation in vitro . J. Mol. Med. Berl. 101 (11), 1437–1448. 10.1007/s00109-023-02374-9 - DOI - PMC - PubMed
-
- Albrecht M., Hummitzsch L., Rusch R., Heß K., Steinfath M., Cremer J., et al. (2023b). Characterization of large extracellular vesicles (L-EV) derived from human regulatory macrophages (Mreg): novel mediators in wound healing and angiogenesis? J. Transl. Med. 21 (1), 61. 10.1186/s12967-023-03900-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

