Effect of methotrexate/dexamethasone combination on epithelial-mesenchymal transition and inflammation gene expression of human RPE cells in-vitro
- PMID: 40371355
- PMCID: PMC12075955
- DOI: 10.3389/fphar.2025.1569703
Effect of methotrexate/dexamethasone combination on epithelial-mesenchymal transition and inflammation gene expression of human RPE cells in-vitro
Abstract
Introduction: RPE cells serve as an experimental model for studying a retinal disease called proliferative vitreoretinopathy (PVR). The pathological background of PVR involves uncontrolled cell proliferation, increased inflammation, and enhanced epithelial-mesenchymal transition (EMT), which have been the focus of various research studies. The present study aimed to explore the effects of combination therapy using methotrexate (MTX) and dexamethasone (DEXA) on the expression of genes involved in apoptosis, inflammation and EMT in retinal pigment epithelial (RPE) cells.
Methods: Our study design comprised two sets of experiments. First, we assessed the effect of MTX serial dilutions (0.5x, x, 2x, and 4x, where x = 100 μg/mL) on RPE cells to determine the optimal concentration of MTX that promotes apoptosis-related gene expression without altering inflammatory-related gene expression. Second, we investigated the influence of MTX (at the selected dose) alone or in combination with DEXA (50 μg/mL) on apoptosis, inflammation, and EMT-related gene expression in RPE cells at the transcriptional level.
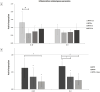
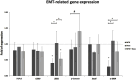
Results: Treatment with 100 μg/mL MTX demonstrated a pro-apoptotic effect according to the expression level of BAX and BCL-2 in RPE cells. The combination of MTX (100 μg/mL) and DEXA significantly reduced the expression of inflammation-related genes (IL-1b, IL-6), indicating a synergistic anti-inflammatory effect. However, there was no significant effect on the expression of genes related to EMT (TGF-β, CD90, β-Catenin, Snail), except for a partial neutralization of the reducing effect of MTX on ZEB1 and α-SMA genes.
Discussion: Our study highlighted the potential pro-apoptotic effect of MTX (at 100 μg/mL) on RPE cells and the synergistic anti-inflammatory impact of MTX/DEXA combination therapy. Nevertheless, this combination did not significantly affect genes associated with EMT. Further research is required to elucidate the clinical implications of these findings in the management of PVR.
Keywords: EMT; PVR; RPE; apoptosis; dexamethasone; inflammation; methotrexate.
Copyright © 2025 Sanie-Jahromi, Ravankhah, Shafi Khani, Razavizadegan and Nowroozzadeh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abrams G., Glazer L. (1993). “Proliferative vitreoretinopathy,” in Practical atlas of retinal disease and therapy. New York: Raven Press Ltd, 279–297.
LinkOut - more resources
Full Text Sources
Research Materials

