Sulindac exhibits anti-proliferative and anti-invasive effects and enhances the sensitivity to paclitaxel in ovarian cancer
- PMID: 40371356
- PMCID: PMC12075207
- DOI: 10.3389/fphar.2025.1520771
Sulindac exhibits anti-proliferative and anti-invasive effects and enhances the sensitivity to paclitaxel in ovarian cancer
Abstract
Objective: Chronic inflammation is a key contributor to carcinogenesis, progression, and chemoresistance in ovarian cancer, making inflammatory pathways a logical therapeutic target for the treatment of this disease. Sulindac, a commonly used non-steroidal anti-inflammatory drug, has demonstrated anti-proliferative and anti-invasive effects on several preclinical models of cancer. In this study, we investigated the antitumorigenic effects of sulindac in human ovarian cancer cell lines and a transgenic mouse model of ovarian cancer (KpB).
Methods: MTT and colony formation assays were used to evaluate cell proliferation. Cell cycle was detected by Cellometer. ELISA assays were conducted to evaluate the changes of cellular stress, apoptosis and adhesion, while invasion was determined by wound healing assay. Protein expression was examined through Western blotting and immunohistochemistry.
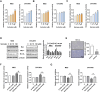
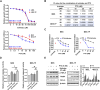
Results: Our results demonstrated that sulindac significantly inhibited cell proliferation, induced cellular stress and apoptosis, caused G1 phase cell cycle arrest, and reduced cell invasion, and suppressed Cox-2 and NF-κB pathways in the MES and OVCAR5 cell lines. Inhibition of cellular stress by N-acetylcysteine partially reversed the anti-proliferative and anti-invasive effects of sulindac. The combination of sulindac and paclitaxel produced synergistic effects in inhibiting cell growth in both paclitaxel sensitive and resistant MES cells. Treatment with sulindac for 4 weeks effectively reduced tumor growth, improved serum levels of inflammatory cytokines and chemokines, and reduced the expression of Cox-2 of ovarian tumors in KpB mice compared with untreated mice.
Conclusions: These findings provide support for the development of clinical trials repurposing sulindac in the treatment of OC, possibly in combination with paclitaxel.
Keywords: cell proliferation; invasion; ovarian cancer; paclitaxel; sulindac; synergy.
Copyright © 2025 Chen, Kong, Shen, Sinha, Haag, Deng, Zhang, John, Sun, Zhou and Bae-Jump.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer HL declared a shared affiliation with the authors WK and BD at the time of review.
Figures






Similar articles
-
Synergistic cytotoxic effect of sulindac and pyrrolidine dithiocarbamate against ovarian cancer cells.Oncol Rep. 2012 Apr;27(4):1245-50. doi: 10.3892/or.2012.1639. Epub 2012 Jan 16. Oncol Rep. 2012. PMID: 22266802 Free PMC article.
-
NSAID-activated gene 1 mediates pro-inflammatory signaling activation and paclitaxel chemoresistance in type I human epithelial ovarian cancer stem-like cells.Oncotarget. 2016 Nov 1;7(44):72148-72166. doi: 10.18632/oncotarget.12355. Oncotarget. 2016. PMID: 27708225 Free PMC article.
-
Ipatasertib exhibits anti‑tumorigenic effects and enhances sensitivity to paclitaxel in endometrial cancer in vitro and in vivo.Int J Oncol. 2023 Sep;63(3):103. doi: 10.3892/ijo.2023.5551. Epub 2023 Jul 28. Int J Oncol. 2023. PMID: 37503790 Free PMC article.
-
Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer.Am J Transl Res. 2016 Oct 15;8(10):4265-4277. eCollection 2016. Am J Transl Res. 2016. PMID: 27830010 Free PMC article.
-
Exisulind: Aptosyn, FGN 1, Prevatac, sulindac sulfone.Drugs R D. 2004;5(4):220-6. doi: 10.2165/00126839-200405040-00007. Drugs R D. 2004. PMID: 15230629 Review.
References
-
- Altinoz M. A., Korkmaz R. (2004). NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma 51 (4), 239–247. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

